METTL3/miR-192-5p/SCD1 Axis Regulates Lipid Metabolism to Affect T Cell Differentiation in Asthma
- PMID: 39867638
- PMCID: PMC11769594
- DOI: 10.1155/mi/4955849
METTL3/miR-192-5p/SCD1 Axis Regulates Lipid Metabolism to Affect T Cell Differentiation in Asthma
Abstract
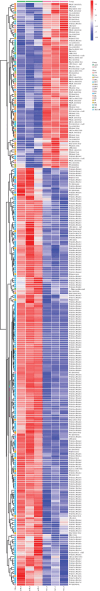
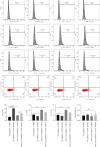
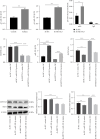
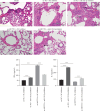
Background: This study aimed to explore the mechanisms underlying T-cell differentiation in asthma. Methods and Results: Flow cytometry was performed to detect Th cells. LC-MS/MS was performed to assess lipid metabolism. HE staining was performed to assess the pathological changes of the lung tissues. ELISA was performed to detect cytokine levels. The results of quantitative real-time polymerase chain reaction (qRT-PCR) and western blot showed that miR-192-5p expression was decreased, while SCD1 expression was increased in CD4+T cells isolated from the peripheral blood of children with asthma. The dual luciferase reporter assay determined the direct interaction between miR-192-5p and SCD1. MiR-192-5p inhibitor reduced ASCL3 and PPARα, increased FASN and SREBP1c mRNA expression and protein levels in mouse spleen CD4+T cells, and elevated Th2 and Th17 cells, but these effects were reversed by the SCD1 inhibitor. Oleic acid (OA) reduced Th1 cells and increased Th2 and Th17 cells in mouse spleen CD4+T cells treated with an SCD1 inhibitor. Additionally, pri-miR-192-5p expression was increased in CD4+T cells isolated from the peripheral blood of asthmatic children, and the deletion of METTL3 upregulated pri-miR-192-5p expression in an m6A-dependent manner. MiR-192-5p mimic and inhibitor both reversed miR-192-5p and SCD1 expression affected by overexpression or deletion of METTL3, both in vivo and in vitro. Furthermore, METTL3 overexpression attenuated lung inflammation, elevated Th1 cells, and reduced Th2 and Th17 cells in CD4+T cells isolated from the peripheral blood of asthmatic mice. These effects were reversed by the miR-192-5p inhibitor. Conclusion: These results suggest that METTL3/miR-192-5p/SCD1 axis regulates lipid metabolism and affects T cell differentiation, thus affecting asthma progression. This study may provide novel insights into the pathogenesis of asthma and a new treatment strategy.
Keywords: METTL3; SCD1; T cell differentiation; asthma; lipid metabolism; miR-192-5p.
Copyright © 2025 Zhengrong Chen et al. Mediators of Inflammation published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
miR-212-5p suppresses lipid accumulation by targeting FAS and SCD1.J Mol Endocrinol. 2017 Oct;59(3):205-217. doi: 10.1530/JME-16-0179. Epub 2017 Jun 30. J Mol Endocrinol. 2017. PMID: 28667176
-
[Spleen-derived CD4+ T cells of asthmatic mice promote M2 polarization of macrophages in vitro].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2019 Apr;35(4):289-295. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2019. PMID: 31167686 Chinese.
-
LDHA- Mediated Histone Lactylation Promotes the Nonalcoholic Fatty Liver Disease Progression Through Targeting The METTL3/ YTHDF1/SCD1 m6A Axis.Physiol Res. 2024 Dec 31;73(6):985-999. doi: 10.33549/physiolres.935289. Physiol Res. 2024. PMID: 39903889 Free PMC article.
-
miR-155 promotes Th17 differentiation by targeting FOXP3 to aggravate inflammation in MRSA pneumonia.Cytokine. 2024 Aug;180:156662. doi: 10.1016/j.cyto.2024.156662. Epub 2024 Jun 1. Cytokine. 2024. PMID: 38824863
-
The Effect of Lipid Metabolism on CD4+ T Cells.Mediators Inflamm. 2021 Jan 5;2021:6634532. doi: 10.1155/2021/6634532. eCollection 2021. Mediators Inflamm. 2021. PMID: 33505215 Free PMC article. Review.
References
-
- Rehman A., Amin F., Sadeeqa S. Prevalence of Asthma and Its Management: A Review. JPMA. The Journal of the Pakistan Medical Association . 2018;68(12):1823–1827. - PubMed
-
- Ahmadian-Elmi M., Bidmeshki Pour A., Naghavian R., et al. miR-27a and miR-214 Exert Opposite Regulatory Roles in Th17 Differentiation via Mediating Different Signaling Pathways in Peripheral Blood CD4+T Lymphocytes of Patients With Relapsing-Remitting Multiple Sclerosis. Immunogenetics . 2016;68(1):43–54. doi: 10.1007/s00251-015-0881-y. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

