Lower incidence of hepatocellular carcinoma with tenofovir alafenamide in chronic hepatitis B: Evidence from a large-scale cohort
- PMID: 39867683
- PMCID: PMC11762169
- DOI: 10.1016/j.jhepr.2024.101268
Lower incidence of hepatocellular carcinoma with tenofovir alafenamide in chronic hepatitis B: Evidence from a large-scale cohort
Abstract
Background & aims: Tenofovir alafenamide (TAF) lacks extensive research regarding its impact on hepatocellular carcinoma (HCC). This study evaluated and compared the effects of TAF, tenofovir disoproxil fumarate (TDF), and entecavir (ETV) on HCC incidence using nationwide claim data.
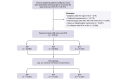
Methods: In total, 75,816 patients with treatment-naïve HBV were included in the study and divided into TAF (n = 25,680), TDF (n = 26,954), and ETV (n = 23,182) groups after exclusions. Propensity score matching (1:1:1) resulted in 17,537 patients per group. HCC incidence rates were compared among the groups.
Results: Before matching, the incidence of HCC was significantly lower in the TAF group compared with the TDF and ETV groups (11.47 vs. 15.04 and 14.24 per 1,000 person-years). The incidence rate ratio (IRR) for TDF was 1.31 (1.19-1.44) and for ETV was 1.24 (1.12-1.37). Before matching, the TAF group had a significantly lower HCC compared with TDF and ETV in both patients with and without cirrhosis. After matching, the TAF group had a lower HCC incidence compared with the TDF group (12.38 vs. 15.39, IRR 1.24, p <0.001) but not with ETV group (IRR 1.08, p = 0.219). In patients with cirrhosis, TAF had lower HCC incidence compared with TDF and ETV (30.25 vs. 39.56 and 38.51, respectively). In patients without cirrhosis, the TAF group had a lower HCC incidence compared with the TDF group (IRR 1.19, p = 0.030) but not the ETV group (IRR 0.85, p = 0.066). Cox regression analysis showed that the TAF group had a significantly lower HCC incidence compared with the TDF (hazard ratio 1.335, p <0.001) and ETV groups (hazard ratio 1.162, p = 0.011), after adjusting for age, gender, and cirrhosis status.
Conclusions: The TAF group consistently demonstrated a lower incidence of HCC compared with the TDF and ETV groups, especially in patients with cirrhosis.
Impact and implications: This work aimed to fill the knowledge gap regarding the comparative efficacy of tenofovir alafenamide (TAF), tenofovir disoproxil fumarate (TDF), and entecavir (ETV) in reducing the incidence of hepatocellular carcinoma (HCC) in patients with chronic HBV. The results are particularly crucial for healthcare providers and policymakers, because they highlight the significantly lower incidence of HCC associated with TAF, especially in patients with cirrhosis. These results suggest TAF as a preferable antiviral therapy option to mitigate HCC risk, thus influencing clinical decision-making and healthcare guidelines. From a practical perspective, these findings can guide physicians in prescribing more effective treatments, assist researchers in designing further studies to explore the mechanisms behind the effectiveness of TAF, and inform policymakers to craft healthcare policies that optimize patient outcomes while considering potential limitations, such as the observational nature of the study and residual confounding factors.
Keywords: Antiviral therapy; Chronic HBV; Cirrhosis; Comparative efficacy; Healthcare data analysis.
© 2024 The Author(s).
Conflict of interest statement
Y.S.K. previously served as an advisor for Gilead and BMS, but currently has no affiliation with either company. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures



References
-
- Sung H., Ferlay J., Siegel R.L., et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

