Molecular Epidemiology of Type F Clostridium perfringens Among Diarrheal Patients and Virulence-Resistance Dynamics - 11 Provinces, China, 2024
- PMID: 39867821
- PMCID: PMC11757904
- DOI: 10.46234/ccdcw2025.013
Molecular Epidemiology of Type F Clostridium perfringens Among Diarrheal Patients and Virulence-Resistance Dynamics - 11 Provinces, China, 2024
Abstract
Introduction: Type F Clostridium perfringens (C. perfringens) represents a significant pathogen in human gastrointestinal diseases, primarily through its cpe gene encoding C. perfringens enterotoxin (CPE). This investigation examined the prevalence, antimicrobial resistance patterns, and genetic characteristics of Type F C. perfringens within the Chinese population.
Methods: The study analyzed 2,068 stool samples collected from 11 provincial hospitals in 2024. Antimicrobial susceptibility testing was conducted following Clinical & Laboratory Standards Institute (CLSI) guidelines, while whole-genome sequencing provided detailed genetic profiles. Evolutionary relationships and clonal transmission patterns were investigated through phylogenetic and genetic environment analyses.
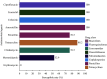
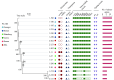
Results: The prevalence of Type F C. perfringens was 2.38%, with isolates predominantly identified in human clinical samples and higher detection rates in gastroenterology departments. Notably, 47.1% of isolates demonstrated high resistance to metronidazole, while all exhibited intermediate resistance to erythromycin. Phylogenetic analysis revealed high similarity among isolates from patients within the same province (single-nucleotide polymorphism (SNPs)<100), and genetic environment analysis indicated potential horizontal gene transfer between animal and human strains.
Conclusions: This investigation predominantly identified Type F C. perfringens in human clinical cases, with sporadic detection in pets and food products. These findings highlight the emergence of Type F C. perfringens outbreaks among diarrheal patients, emphasizing the necessity for targeted interventions as virulence factors increase.
Keywords: Type F Clostridium perfringens; antimicrobial resistance; diarrheal patients; virulence factors.
Copyright © 2025 by Chinese Center for Disease Control and Prevention.
Conflict of interest statement
No conflicts of interest.
Figures

Similar articles
-
Prevalence and Genetic Diversity of Clostridium perfringens Isolates in Hospitalized Diarrheal Patients from Central China.Infect Drug Resist. 2021 Nov 15;14:4783-4793. doi: 10.2147/IDR.S338593. eCollection 2021. Infect Drug Resist. 2021. PMID: 34815676 Free PMC article.
-
Heat resistance differences are common between both vegetative cells and spores of Clostridium perfringens type F isolates carrying a chromosomal vs plasmid-borne enterotoxin gene.Appl Environ Microbiol. 2024 Oct 23;90(10):e0091424. doi: 10.1128/aem.00914-24. Epub 2024 Sep 18. Appl Environ Microbiol. 2024. PMID: 39291987 Free PMC article.
-
Large-Scale Genomic Analyses and Toxinotyping of Clostridium perfringens Implicated in Foodborne Outbreaks in France.Front Microbiol. 2019 Apr 17;10:777. doi: 10.3389/fmicb.2019.00777. eCollection 2019. Front Microbiol. 2019. PMID: 31057505 Free PMC article.
-
Enterotoxigenic Clostridium perfringens: detection and identification.Microbes Environ. 2012;27(4):343-9. doi: 10.1264/jsme2.me12002. Epub 2012 Apr 14. Microbes Environ. 2012. PMID: 22504431 Free PMC article. Review.
-
Prevalence, molecular characterization, and antimicrobial resistance profile of Clostridium perfringens from India: A scoping review.Anaerobe. 2022 Oct;77:102639. doi: 10.1016/j.anaerobe.2022.102639. Epub 2022 Sep 13. Anaerobe. 2022. PMID: 36108893
Cited by
-
Prevalence and toxin gene profiles of Clostridium perfringens in diarrheic dogs and cats in Korea: a retrospective analysis.J Vet Sci. 2025 Jul;26(4):e52. doi: 10.4142/jvs.24361. J Vet Sci. 2025. PMID: 40765232 Free PMC article.
References
-
- Kiu R, Caim S, Painset A, Pickard D, Swift C, Dougan G, et al Phylogenomic analysis of gastroenteritis-associated Clostridium perfringens in England and Wales over a 7-year period indicates distribution of clonal toxigenic strains in multiple outbreaks and extensive involvement of enterotoxin-encoding (CPE) plasmids. Microb Genom. 2019;5(10):e000297. doi: 10.1099/mgen.0.000297. - DOI - PMC - PubMed
-
- Zhao FL, Gao X, Zhen BJ, Zhang P, Luo YX, Wang RP, et al Laboratory detection and analysis of a suspected diarrhea outbreak caused by Clostridium perfringens. Chin J Food Hyg. 2023;35(7):1109–13. doi: 10.13590/j.cjfh.2023.07.021. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
