Inhibition of hippocampal interleukin-6 receptor-evoked signalling normalises long-term potentiation in dystrophin-deficient mdx mice
- PMID: 39867844
- PMCID: PMC11762146
- DOI: 10.1016/j.bbih.2024.100935
Inhibition of hippocampal interleukin-6 receptor-evoked signalling normalises long-term potentiation in dystrophin-deficient mdx mice
Abstract
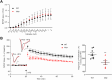
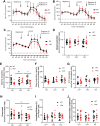
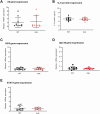
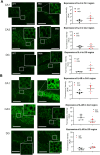
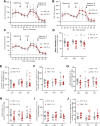
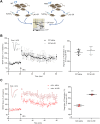
Duchenne muscular dystrophy (DMD), an X-linked neuromuscular disorder, characterised by progressive immobility, chronic inflammation and premature death, is caused by the loss of the mechano-transducing signalling molecule, dystrophin. In non-contracting cells, such as neurons, dystrophin is likely to have a functional role in synaptic plasticity, anchoring post-synaptic receptors. Dystrophin-expressing hippocampal neurons are key to cognitive functions such as emotions, learning and the consolidation of memories. In the context of disease-induced chronic inflammation, we have explored the role of the pleiotropic cytokine, interleukin (IL)-6 in hippocampal dysfunction using immunofluorescence, electrophysiology and metabolic measurements in dystrophic mdx mice. Hippocampal long-term potentiation (LTP) of the Schaffer collateral-CA1 projections was suppressed in mdx slices. Given the importance of mitochondria-generated ATP in synaptic plasticity, reduced maximal respiration in the CA1 region may impact upon this process. Consistent with a role for IL-6 in this observation, early LTP was suppressed in dystrophin-expressing wildtype slices exposed to IL-6. In dystrophic mdx mice, exposure to IL-6 suppressed mitochondrial-mediated basal metabolism in CA1, CA3 and DG hippocampal regions. Furthermore, blocking IL-6-mediated signalling by administering neutralising monoclonal IL-6 receptor antibodies intrathecally, normalised LTP in mdx mice. The impact of dystrophin loss from the hippocampus was associated with modified cellular bioenergetics, which underpin energy-driven processes such as the induction of LTP. The additional challenge of pathophysiological levels of IL-6 resulted in altered cellular bioenergetics, which may be key to cognitive deficits associated with the loss of dystrophin.
Keywords: Bioenergetics; Duchenne muscular dystrophy; Dystrophin; Hippocampus; Interleukin-6; Long-term potentiation.
© 2024 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dervla O'Malley reports financial support was provided by 10.13039/501100001590Health Research Board Ireland. Dervla O'Malley reports equipment, drugs, or supplies was provided by Chugai Pharmaceutical Co., Ltd, Tokyo, Japan. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Neuronal hyperexcitability in dystrophin-deficient mdx hippocampal neurons: the importance of interleukin-6 and GABAergic regulation.Sci Rep. 2025 May 30;15(1):18984. doi: 10.1038/s41598-025-00880-z. Sci Rep. 2025. PMID: 40447687 Free PMC article.
-
Impaired long-term spatial and recognition memory and enhanced CA1 hippocampal LTP in the dystrophin-deficient Dmd(mdx) mouse.Neurobiol Dis. 2004 Oct;17(1):10-20. doi: 10.1016/j.nbd.2004.05.004. Neurobiol Dis. 2004. PMID: 15350961
-
Facilitated CA1 hippocampal synaptic plasticity in dystrophin-deficient mice: role for GABAA receptors?Hippocampus. 2002;12(6):713-7. doi: 10.1002/hipo.10068. Hippocampus. 2002. PMID: 12542223
-
Interleukin-6: A neuro-active cytokine contributing to cognitive impairment in Duchenne muscular dystrophy?Cytokine. 2020 Sep;133:155134. doi: 10.1016/j.cyto.2020.155134. Epub 2020 May 23. Cytokine. 2020. PMID: 32454436 Review.
-
Brain function in Duchenne muscular dystrophy.Brain. 2002 Jan;125(Pt 1):4-13. doi: 10.1093/brain/awf012. Brain. 2002. PMID: 11834588 Review.
Cited by
-
Astrocyte proliferation in the hippocampal dentate gyrus is suppressed across the lifespan of dystrophin-deficient mdx mice.Exp Physiol. 2025 Apr;110(4):585-598. doi: 10.1113/EP092150. Epub 2025 Jan 10. Exp Physiol. 2025. PMID: 39792584 Free PMC article.
-
Neuronal hyperexcitability in dystrophin-deficient mdx hippocampal neurons: the importance of interleukin-6 and GABAergic regulation.Sci Rep. 2025 May 30;15(1):18984. doi: 10.1038/s41598-025-00880-z. Sci Rep. 2025. PMID: 40447687 Free PMC article.
References
-
- Anderson J.L., Head S.I., Rae C., Morley J.W. Brain function in Duchenne muscular dystrophy. Brain. 2002;125:4–13. - PubMed
-
- Banks W.A., Kastin A.J., Gutierrez E.G. Penetration of interleukin-6 across the murine blood-brain barrier. Neurosci. Lett. 1994;179:53–56. - PubMed
-
- Bliss T.V., Collingridge G.L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

