Esketamine at a Clinical Dose Attenuates Cerebral Ischemia/Reperfusion Injury by Inhibiting AKT Signaling Pathway to Facilitate Microglia M2 Polarization and Autophagy
- PMID: 39867864
- PMCID: PMC11760763
- DOI: 10.2147/DDDT.S504179
Esketamine at a Clinical Dose Attenuates Cerebral Ischemia/Reperfusion Injury by Inhibiting AKT Signaling Pathway to Facilitate Microglia M2 Polarization and Autophagy
Abstract
Purpose: This study aimed to assess the protective effect of a clinical dose esketamine on cerebral ischemia/reperfusion (I/R) injury and to reveal the potential mechanisms associated with microglial polarization and autophagy.
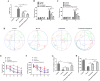
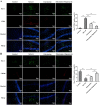
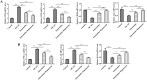
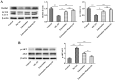
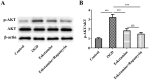
Methods: Experimental cerebral ischemia was induced by middle cerebral artery occlusion (MCAO) in adult rats and simulated by oxygen-glucose deprivation (OGD) in BV-2 microglial cells. Neurological and sensorimotor function, cerebral infarct volume, histopathological changes, mitochondrial morphological changes, and apoptosis of ischemic brain tissues were assessed in the presence or absence of esketamine and the autophagy inducer rapamycin. The expression of biomarkers related to microglial M1 and M2 phenotypes in the ischemic brain tissues was determined by immunofluorescence staining and RT-qPCR, and the expression of proteins associated with autophagy and the AKT signaling pathway in the ischemic brain tissues was assayed by Western blotting.
Results: Esketamine alone and esketamine combined with rapamycin alleviated neurological impairment, improved sensorimotor function, decreased cerebral infarct volume, and mitigated tissue injury in the MCAO rats. Importantly, esketamine promoted microglial phenotypic transition from M1 to M2 in both the MCAO rats and the OGD-treated BV-2 microglia, induced autophagy, and inactivated AKT signaling. Furthermore, the effects of esketamine were enhanced by addition of autophagy inducer rapamycin.
Conclusion: Esketamine at a clinical dose attenuates cerebral I/R injury by inhibiting AKT signaling pathway to facilitate microglial M2 polarization and autophagy. Furthermore, esketamine combined autophagy inducer can provide an improved protection against cerebral I/R injury. Thus, this study provides new insights into the neuroprotective mechanisms of esketamine and the potential therapeutic strategies of cerebral I/R injury.
Keywords: autophagy; cerebral ischemia/reperfusion injury; esketamine; ischemic stroke; microglia polarization.
© 2025 Gao et al.
Conflict of interest statement
The authors declare no competing interests in this work.
Figures



Similar articles
-
Polyphyllin I alleviates neuroinflammation after cerebral ischemia-reperfusion injury via facilitating autophagy-mediated M2 microglial polarization.Mol Med. 2024 May 14;30(1):59. doi: 10.1186/s10020-024-00828-5. Mol Med. 2024. PMID: 38745316 Free PMC article.
-
Shikonin attenuates cerebral ischemia/reperfusion injury via inhibiting NOD2/RIP2/NF-κB-mediated microglia polarization and neuroinflammation.J Stroke Cerebrovasc Dis. 2024 Jun;33(6):107689. doi: 10.1016/j.jstrokecerebrovasdis.2024.107689. Epub 2024 Mar 26. J Stroke Cerebrovasc Dis. 2024. PMID: 38527567
-
Lipoxin A4 regulates microglial M1/M2 polarization after cerebral ischemia-reperfusion injury via the Notch signaling pathway.Exp Neurol. 2021 May;339:113645. doi: 10.1016/j.expneurol.2021.113645. Epub 2021 Feb 15. Exp Neurol. 2021. PMID: 33600815
-
Loureirin B protects against cerebral ischemia/reperfusion injury through modulating M1/M2 microglial polarization via STAT6 / NF-kappaB signaling pathway.Eur J Pharmacol. 2023 Aug 15;953:175860. doi: 10.1016/j.ejphar.2023.175860. Epub 2023 Jun 16. Eur J Pharmacol. 2023. PMID: 37331681 Review.
-
PI3K/AKT pathway: A potential therapeutic target in cerebral ischemia-reperfusion injury.Eur J Pharmacol. 2025 Jul 5;998:177505. doi: 10.1016/j.ejphar.2025.177505. Epub 2025 Mar 19. Eur J Pharmacol. 2025. PMID: 40118329 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

