The role of M1/M2 macrophage polarization in the pathogenesis of obesity-related kidney disease and related pathologies
- PMID: 39867890
- PMCID: PMC11758166
- DOI: 10.3389/fimmu.2024.1534823
The role of M1/M2 macrophage polarization in the pathogenesis of obesity-related kidney disease and related pathologies
Abstract
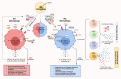
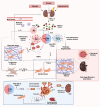
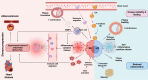
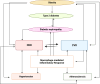
Obesity is a rapidly growing health problem worldwide, affecting both adults and children and increasing the risk of chronic diseases such as type 2 diabetes, hypertension and cardiovascular disease (CVD). In addition, obesity is closely linked to chronic kidney disease (CKD) by either exacerbating diabetic complications or directly causing kidney damage. Obesity-related CKD is characterized by proteinuria, lipid accumulation, fibrosis and glomerulosclerosis, which can gradually impair kidney function. Among the immune cells of the innate and adaptive immune response involved in the pathogenesis of obesity-related diseases, macrophages play a crucial role in the inflammation associated with CKD. In obese individuals, macrophages enter a pro-inflammatory state known as M1 polarization, which contributes to chronic inflammation. This polarization promotes tissue damage, inflammation and fibrosis, leading to progressive loss of kidney function. In addition, macrophage-induced oxidative stress is a key feature of CKD as it also promotes cell damage and inflammation. Macrophages also contribute to insulin resistance in type 2 diabetes by releasing inflammatory molecules that impair glucose metabolism, complicating the management of diabetes in obese patients. Hypertension and atherosclerosis, which are often associated with obesity, also contribute to the progression of CKD via immune and inflammatory pathways. Macrophages influence blood pressure regulation and contribute to vascular inflammation, particularly via the renin-angiotensin system. In atherosclerosis, macrophages accumulate in arterial plaques, leading to chronic inflammation and plaque instability, which may increase the risk of CVD in CKD patients. This review focuses on the involvement of macrophages in CKD and highlights their role as a critical link between CKD and other pathologies. Targeting macrophage polarization and the ensuing macrophage-induced inflammation could be an effective therapeutic strategy for CKD and related diseases and improve outcomes for patients with obesity-related kidney disease.
Keywords: M1; M2; cardiovascular disease; chronic kidney disease; macrophages; obesity.
Copyright © 2025 Dousdampanis, Aggeletopoulou and Mouzaki.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
Similar articles
-
Macrophage polarization in innate immune responses contributing to pathogenesis of chronic kidney disease.BMC Nephrol. 2020 Jul 13;21(1):270. doi: 10.1186/s12882-020-01921-7. BMC Nephrol. 2020. PMID: 32660446 Free PMC article. Review.
-
Friend or foe? The role of SIRT6 on macrophage polarized to M2 subtype in acute kidney injury to chronic kidney disease.Ren Fail. 2025 Dec;47(1):2482121. doi: 10.1080/0886022X.2025.2482121. Epub 2025 Apr 22. Ren Fail. 2025. PMID: 40260529 Free PMC article. Review.
-
Progranulin enhances M2 macrophage polarization and renal fibrosis by modulating autophagy in chronic kidney disease.Cell Mol Life Sci. 2025 Apr 28;82(1):186. doi: 10.1007/s00018-025-05716-7. Cell Mol Life Sci. 2025. PMID: 40293508 Free PMC article.
-
Mitochondria in monocytes and macrophages-implications for translational and basic research.Int J Biochem Cell Biol. 2014 Aug;53:202-207. doi: 10.1016/j.biocel.2014.05.019. Epub 2014 May 23. Int J Biochem Cell Biol. 2014. PMID: 24863362 Free PMC article.
-
Emodin inhibits M1 macrophage activation that related to acute and chronic kidney injury through EGFR/MAPK pathway.Funct Integr Genomics. 2024 Jul 30;24(4):131. doi: 10.1007/s10142-024-01407-x. Funct Integr Genomics. 2024. PMID: 39078513
Cited by
-
Immunometabolic Interactions in Obesity: Implications for Therapeutic Strategies.Biomedicines. 2025 Jun 10;13(6):1429. doi: 10.3390/biomedicines13061429. Biomedicines. 2025. PMID: 40564149 Free PMC article. Review.
-
Biomolecule-functionalized dental implant surfaces: Towards augmenting soft tissue integration.Bioact Mater. 2025 Jul 26;53:540-590. doi: 10.1016/j.bioactmat.2025.07.005. eCollection 2025 Nov. Bioact Mater. 2025. PMID: 40755849 Free PMC article. Review.
-
Targeting ion channel networks in diabetic kidney disease: from molecular crosstalk to precision therapeutics and clinical innovation.Front Med (Lausanne). 2025 Jun 26;12:1607701. doi: 10.3389/fmed.2025.1607701. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40641971 Free PMC article. Review.
-
Macrophage Depletion Alleviates Immunosenescence in Diabetic Kidney by Modulating GDF-15 and Klotho.Int J Mol Sci. 2025 Apr 23;26(9):3990. doi: 10.3390/ijms26093990. Int J Mol Sci. 2025. PMID: 40362229 Free PMC article.
-
Anti-Inflammatory Actions of Plant-Derived Compounds and Prevention of Chronic Diseases: From Molecular Mechanisms to Applications.Int J Mol Sci. 2025 May 28;26(11):5206. doi: 10.3390/ijms26115206. Int J Mol Sci. 2025. PMID: 40508016 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

