Development and external validation of a model to predict recurrence in patients with non-muscle invasive bladder cancer
- PMID: 39867903
- PMCID: PMC11757240
- DOI: 10.3389/fimmu.2024.1467527
Development and external validation of a model to predict recurrence in patients with non-muscle invasive bladder cancer
Abstract
Background: Most patients initially diagnosed with non-muscle invasive bladder cancer (NMIBC) still have frequent recurrence after urethral bladder tumor electrodesiccation supplemented with intravesical instillation therapy, and their risk of recurrence is difficult to predict. Risk prediction models used to predict postoperative recurrence in patients with NMIBC have limitations, such as a limited number of included cases and a lack of validation. Therefore, there is an urgent need to develop new models to compensate for the shortcomings and potentially provide evidence for predicting postoperative recurrence in NMIBC patients.
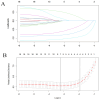
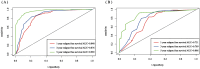
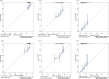
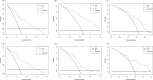
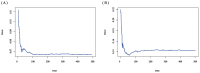
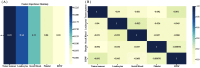
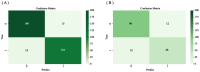
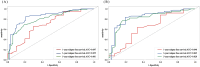
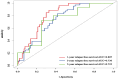
Methods: Clinicopathologic characteristics and follow-up data were retrospectively collected from 556 patients with NMIBC who underwent transurethral resection of bladder tumors by electrocautery (TURBT) from January 2014 to December 2023 at the Affiliated Hospital of Zunyi Medical University and 167 patients with NMIBC who underwent the same procedure from January 2018 to April 2024 at the Third Affiliated Hospital of Zunyi Medical University. Independent risk factors affecting the recurrence of NMIBC were screened using the least absolute shrinkage and selection operator (Lasso) and Cox regression analysis. Cox risk regression models and randomized survival forest (RSF) models were developed. The optimal model was selected by comparing the area under the curve (AUC) of the working characteristics of the subjects in both and presented as a column-line graph.
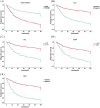
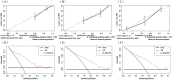
Results: The study included data from 566 patients obtained from the affiliated hospital of Zunyi Medical University and 167 patients obtained from the third affiliated hospital of Zunyi Medical University. Tumor number, urine leukocytes, urine occult blood, platelets, and red blood cell distribution width were confirmed as independent risk factors predicting RFS by Lasso-Cox regression analysis. The Cox proportional risk regression model and RSF model were constructed based on Lasso, which showed good predictive efficacy in both training and validation sets, especially the traditional Cox proportional risk regression model. In addition, the discrimination, consistency, and clinical utility of the column-line graph were assessed using C-index, area under the curve (AUC), calibration curve, and decision curve analysis (DCA). Patients at high risk of recurrence can be identified early based on risk stratification.
Conclusion: Internal and external validation has demonstrated that the model is highly discriminative and stable and can be used to assess the risk of early recurrence in NMIBC patients and to guide clinical decision-making.
Keywords: Lasso-Cox regression; nomogram; non-muscle invasive bladder cancer; random forest; recurrence.
Copyright © 2025 Tang, Fan, Huang, Yang, Yang, Liao, Zuo, Zhang and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Development and external validation of a novel nomogram model for predicting postoperative recurrence-free survival in non-muscle-invasive bladder cancer.Front Immunol. 2022 Nov 15;13:1070043. doi: 10.3389/fimmu.2022.1070043. eCollection 2022. Front Immunol. 2022. PMID: 36458001 Free PMC article.
-
Analysis of Risk Factors for Recurrence after Transurethral Resection of Bladder Tumor in Patients with Non-Muscle Invasive Bladder Cancer: 2-Year Follow-Up Outcomes.Oncology. 2024;102(4):337-342. doi: 10.1159/000533410. Epub 2023 Aug 30. Oncology. 2024. PMID: 37647883 Free PMC article.
-
Novel nomograms to predict recurrence and progression in primary non-muscle-invasive bladder cancer: validation of predictive efficacy in comparison with European Organization of Research and Treatment of Cancer scoring system.World J Urol. 2019 Sep;37(9):1867-1877. doi: 10.1007/s00345-018-2581-3. Epub 2018 Dec 10. World J Urol. 2019. PMID: 30535715
-
Preoperative fluorescence in situ hybridization analysis as a predictor of tumor recurrence in patients with non-muscle invasive bladder cancer: a bi-institutional study.J Transl Med. 2023 Oct 2;21(1):685. doi: 10.1186/s12967-023-04528-2. J Transl Med. 2023. PMID: 37784106 Free PMC article.
-
Metastasis development in non-muscle-invasive bladder cancer.Nat Rev Urol. 2025 Jun;22(6):375-386. doi: 10.1038/s41585-024-00963-y. Epub 2024 Nov 20. Nat Rev Urol. 2025. PMID: 39567681 Review.
References
-
- Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. . Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. (2006) 49:466–5. doi: 10.1016/j.eururo.2005.12.031 - DOI - PubMed
-
- Fernandez-Gomez J, Madero R, Solsona E, Unda M, Martinez-Piñeiro L, Gonzalez M, et al. . Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with bacillus Calmette-Guerin: the CUETO scoring model. J Urol. (2009) 182:2195–203. doi: 10.1016/j.juro.2009.07.016 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

