m5C methylation modification may be an accomplice in colorectal cancer escaping from anti-tumor effects of innate immunity-type I/III interferon
- PMID: 39867908
- PMCID: PMC11757137
- DOI: 10.3389/fimmu.2024.1512353
m5C methylation modification may be an accomplice in colorectal cancer escaping from anti-tumor effects of innate immunity-type I/III interferon
Abstract
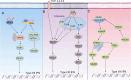
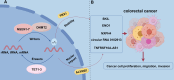
Colorectal cancer (CRC) is one of the most prevalent malignant tumors in the world, and its occurrence and development are closely related to the complex immune regulatory mechanisms. As the first barrier of the body's defense, innate immunity plays a key role in tumor immune surveillance and anti-tumor response, in which type I/III interferon (IFN) is an important mediator with significant antiviral and anti-tumor functions. 5-methylcytosine (m5C) modification of RNA is a key epigenetic regulation that promotes the expression of CRC oncogenes and immune-related genes. It can enhance the proliferation, migration, and invasion of tumor cells by affecting mRNA stability, translation efficiency, and nuclear export. In addition, m5C modification modulates the activity of innate immune signaling pathways and inhibits interferon production and function, further helping tumor cells evade immune surveillance. However, there are insufficient elucidations on the interaction between m5C modification and innate immunity in CRC. In this study, the mechanism of interferon I/III in colorectal cancer was systematically reviewed and explored. This work focused on how m5C modification promotes tumor immune escape by affecting the interferon signaling pathway, thereby providing new diagnostic markers and therapeutic targets for clinical use, and enhancing the immunotherapy efficacy.
Keywords: colorectal cancer; innate immune signaling pathways; m5C methylation; tumor immune escape; type I/III interferon.
Copyright © 2025 Sun, Liu, Jiang and Zhong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

