Roads to remission: evolving treatment concepts in type 2 inflammatory diseases
- PMID: 39867971
- PMCID: PMC11764424
- DOI: 10.1016/j.eclinm.2024.103050
Roads to remission: evolving treatment concepts in type 2 inflammatory diseases
Abstract
Non-communicable diseases (NCDs) characterised by type 2 inflammation, including asthma, allergic rhinitis, chronic rhinosinusitis with nasal polyps, atopic dermatitis, food allergies and eosinophilic esophagitis, are increasing in prevalence worldwide. Currently, there is a major paradigm shift in the management of these diseases, towards the concept of disease modification and the treatment goal remission, regardless of severity and age. Remission as a treatment goal in chronic inflammatory NCDs was first introduced in rheumatoid arthritis, and then adopted in other non-type 2 inflammatory diseases. Among diseases with type 2 Inflammation, this concept is novel and currently most advanced in asthma. This new paradigm has been developed based on a better understanding of the pathophysiology of type 2 inflammation and the advent of highly effective drugs selectively interfering with type 2 pathways. Here, we review the evolution of the new remission concepts in type 2 inflammatory diseases and discuss associated challenges and future research needs.
Funding: None.
Keywords: Disease modification; Remission; Type 2 inflammation.
© 2024 The Author(s).
Conflict of interest statement
ML reports grants for research or clinical trials, paid to his institution, from AstraZeneca, Deutsche Forschungsgemeinschaft (DFG), and GSK; and consulting fees, travel expenses, or honoraria for lectures from ALK, Allergopharma, Apontis, AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, Chiesi, GSK, HAL Allergy, Leti, Novartis, MSD, Sanofi, Stallergenes, Teva. KB reports on grants for research or clinical trials, paid to the institution from Aimmune therapeutics, Nestle, DBV technologies, Novartis, Hipp GmbH and consulting fees, travel expenses, or honoraria for lectures from ALK-Abello Arzneimittel GmbH, Allergopharma, Aimmune therapeutics, Allergy therapeutics, Danone Deutschland GmbH, DBV Technologies, Engelhard Arzneimittel, Novartis Pharma AG, Sanofi, ThermoFisher Scientific, Mylan Germany GmbH, German society of clinical chemistry and laboratory medicine, Stallergenes GmbH, German society of allergology and clinical immunology, German society of pediatric allergology and environmental medicine, Austrian society of children and adolescent medicine, Medical association of German allergologists, European academy of allergology and clinical immunology. LAB reports consulting fees from Allakos, Amgen, Arcutis, Arena Pharmaceuticals, Astra Zeneca, Astria Therapeutics, Evelo Biosciences, Escient Pharma, Galderma, Incyte, Invea Therapeutics, Janssen, LEO Pharma, Merck, Nektar Therapeutics, Novartis, Numab Therapeutics, Pfizer, Rapt Therapeutics, Regeneron Pharmaceuticals Inc., Ribon Therapeutics, Sanofi-Aventis/Genzyme, Sitryx Therapeutics, Stealth BioTherapeutics, Trevi Therapeutics, UCB Pharma, Union therapeutics, and Xencor and research grants from Abbvie, AstraZeneca, Pfizer, Regeneron Pharmaceuticals Inc., and Sanofi. JB reports personal fees from Cipla, Menarini, Mylan, Novartis, Purina, Sanofi-Aventis, Teva, Noucor, KYomed-Innov, Mask-air-SAS. GGB reports consulting fees, travel expenses, or honoraria for lectures from AstraZeneca, Boehringer-Ingelheim, Chiesi, GlaxoSmithKline, Merck Sharp & Dohme, Novartis, Sanofi Regeneron. WF reports consultation and/or speaker fees from Dianosic, GSK, Novartis, Sanofi-Aventis/Regeneron and AstraZeneca. EH reports grants for research or clinical trials, paid to his institution, from the German Ministry of Education and Research (BMBF), InfectoPharm, Wolff, AstraZeneca; and consulting fees, travel expenses, or honoraria for lectures from ALK, Allergopharma, AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, GSK, HAL Allergy, Leti, Novartis, Sanofi, Stallergenes. SL reports research grants from DFG (German Research Foundation), Einstein Foundation, DBV, and consultation and/or speaker fees from Allergopharma, ALK, DBV, GSK, LETI, Leo-Pharma, Lilly, Viatris, Sanofi-Aventis. OP reports grants and/or personal fees and/or travel support from ALK-Abelló, Allergopharma, Stallergenes Greer, HAL Allergy Holding B.V./HAL Allergie GmbH, Bencard Allergie GmbH/Allergy Therapeutics, Laboratorios LETI/LETI Pharma, GlaxoSmithKline, ROXALL Medizin, Novartis, Sanofi-Aventis and Sanofi-Genzyme, Med Update Europe GmbH, streamedup! GmbH, Pohl-Boskamp, Inmunotek S.L., John Wiley and Sons/AS, Paul-Martini-Stiftung (PMS), Regeneron Pharmaceuticals Inc., RG Aerztefortbildung, Institut für Disease Management, Springer GmbH, AstraZeneca, IQVIA Commercial, Ingress Health, Wort&Bild Verlag, Verlag ME, Procter&Gamble, ALTAMIRA, Meinhardt Congress GmbH, Deutsche Forschungsgemeinschaft (DFG), Thieme Verlag, Deutsche AllergieLiga e.V., AeDA, Alfried-Krupp Krankenhaus, Red Maple Trials Inc., Königlich Dänisches Generalkonsulat, Medizinische Hochschule Hannover, ECM Expro&Conference Management, Technical University Dresden, Lilly, Japanese Society of Allergy, Forum für Medizinische Fortbildung, Dustri-Verlag, Pneumolive, ASIT Biotech, LOFARMA, Almirall, Paul-Ehrlich-Institut. HAS reports grant funding to his institution from NIH/NIAID and personal consulting fees from DBV Technologies, N-Fold, Alpina Biotechnology and Siolta, and stock options from DBV Technologies and N-Fold Therapeutics. MWa reports grants for research or clinical trials, paid to his institution, from ALK-Abelló, AstraZeneca, EU, GlaxoSmithKline, Novartis, Regeneron, Sanofi-Aventis, Takeda; and consulting fees, travel expenses, or honoraria for lectures from Allergopharma, ALK-Abelló, AstraZeneca, CSL Behring, Genzyme, GSK, HAL Allergie, Infectopharm, LETI Pharma, Novartis, Regeneron, Sanofi, Stallergenes. TW reports institutional grants or personal fees for lectures or advisory boards from AbbVie, Almirall, Beiersdorf, Eli Lilly, Galderma, Janssen/JNJ, Leo Pharma, Novartis, Pfizer, Sanofi-Regeneron. MWo reports honoraria or consultation fees from Novartis Pharma GmbH, Sanofi-Aventis Deutschland GmbH, DBV Technologies S.A, Aimmune Therapeutics UK Limited, Leo Pharma GmbH, AstraZenceca GmbH, ALK-Abelló Arzneimittel GmbH, Lilly Deutschland GmbH, Kymab Limited, Amgen GmbH, Abbvie Deutschland GmbH & Co. KG, Pfizer Pharma GmbH, Mylan Germany GmbH (A Viatris Company), Boehringer Ingelheim Pharma GmbH & Co. KG, GlaxoSmithKline GmbH & Co. KG, Almirall S. A., Amgen GmbH, Pfizer Deutschland GmbH, Bristol-Myers Squibb GmbH & Co. KG. HR reports grants from Deutsche Lungenzentrum (DZL), Lungenzentrum der Universitäten Gießen und Marburg (UGLMC), MIRACUM-Konsortium, Stiftung Pathobiochemie, Krankenhauspartnerschaftsprogramm DAAD and GIZ, Deutsche Forschungsgemeinschaft (DFG), Bundesministerium für Bildung und Forschung (BMBF), European Union (EU), and onsultation and/or speaker fees from Allergopharma, Novartis, ThermoFisher, Danone, Bencard, Stallergenes, GSK, AstraZeneca, Sterna biologicals. HO, JSS, CT and IHT do not report any conflicts of interest.
Figures
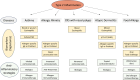
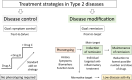
References
-
- Kolkhir P., Akdis C.A., Akdis M., et al. Type 2 chronic inflammatory diseases: targets, therapies and unmet needs. Nat Rev Drug Discov. 2023;22(9):743–767. - PubMed
-
- Lommatzsch M., Brusselle G.G., Canonica G.W., et al. Disease-modifying anti-asthmatic drugs. Lancet. 2022;399(10335):1664–1668. - PubMed
-
- Bieber T. Disease modification in inflammatory skin disorders: opportunities and challenges. Nat Rev Drug Discov. 2023;22(8):662–680. - PubMed
-
- Fokkens W.J., De Corso E., Backer V., et al. EPOS2020/EUFOREA expert opinion on defining disease states and therapeutic goals in CRSwNP. Rhinology. 2024;62(3):287–298. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

