Bioprinting approaches in cardiac tissue engineering to reproduce blood-pumping heart function
- PMID: 39868032
- PMCID: PMC11763539
- DOI: 10.1016/j.isci.2024.111664
Bioprinting approaches in cardiac tissue engineering to reproduce blood-pumping heart function
Abstract
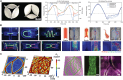
The heart, with its complex structural and functional characteristics, plays a critical role in sustaining life by pumping blood throughout the entire body to supply nutrients and oxygen. Engineered heart tissues have been introduced to reproduce heart functions to understand the pathophysiological properties of the heart and to test and develop potential therapeutics. Although numerous studies have been conducted in various fields to increase the functionality of heart tissue to be similar to reality, there are still many difficulties in reproducing the blood-pumping function of the heart. In this review, we discuss advancements in cells, biomaterials, and biofabrication in cardiac tissue engineering to achieve cardiac models that closely mimic the pumping function. Moreover, we provide insight into future directions by proposing future perspectives to overcome remaining challenges, such as scaling up and biomimetic patterning of blood vessels and nerves through bioprinting.
Keywords: Bioengineering; Biomaterials; Cardiovascular medicine; Tissue engineering.
© 2024 The Authors.
Conflict of interest statement
J.J. is one of the Guest Editors of the Special Issue “Advanced biomanufacturing of cardiovascular tissues” at iScience.
Figures





References
-
- Tortora G.J., Derrickson B. 15th ed. John Wiley & Sons, Inc.; 2014. Principles of Anatomy and Physiology.
Publication types
LinkOut - more resources
Full Text Sources

