This is a preprint.
The ratio of Wnt signaling activity to Sox2 transcription factor levels predicts neuromesodermal fate potential
- PMID: 39868081
- PMCID: PMC11761523
- DOI: 10.1101/2025.01.16.633481
The ratio of Wnt signaling activity to Sox2 transcription factor levels predicts neuromesodermal fate potential
Update in
-
The ratio of Wnt signaling activity to Sox2 transcription factor levels predicts neuromesodermal fate potential.Development. 2025 Nov 15;152(22):dev204661. doi: 10.1242/dev.204661. Epub 2025 Nov 17. Development. 2025. PMID: 41071681
Abstract
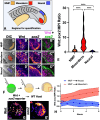
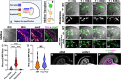
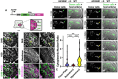
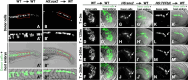
Neuromesodermal progenitors (NMPs) are a vertebrate cell type that contribute descendants to both the spinal cord and the mesoderm. The undifferentiated bipotential NMP state is maintained when both Wnt signaling is active and Sox2 is present. We used transgenic reporter lines to live-image both Wnt activity and Sox2 levels in NMPs and observed a unique cellular ratio in NMPs compared to NMP-derived mesoderm or neural tissue. We used this unique signature to identify the previously unknown anatomical position of a progenitor population that gives rise to the midline tissues of the floor plate of the spinal cord and the mesodermal notochord. Thus, quantification of the active Wnt signaling to Sox2 ratio can be used to predict and identify cells with neuromesodermal potential. We also developed the auxin inducible degron 2 system for use in zebrafish to test the temporal role that Sox2 plays during midline formation. We found ectopic Sox2 in the presence of Wnt activity holds cells in the undifferentiated floor plate/notochord progenitor state, and that degradation of the ectopic Sox2 is required for cells to adopt a notochord fate.
Keywords: Auxin inducible degron; Floor plate; Neuromesodermal progenitors; Notochord; Sox2; Wnt; single cell sequencing.
Figures




References
-
- Adikes R.C., Kohrman A.Q., Martinez M.A.Q., Palmisano N.J., Smith J.J., Medwig-Kinney T.N., Min M., Sallee M.D., Ahmed O.B., Kim N., Liu S., Morabito R.D., Weeks N., Zhao Q., Zhang W., Feldman J.L., Barkoulas M., Pani A.M., Spencer S.L., Martin B.L., Matus D.Q., 2020. Visualizing the metazoan proliferation-quiescence decision in vivo. Elife 9 ( 10.7554/eLife.63265). - DOI - PMC - PubMed
-
- Amacher S.L., Kimmel C.B., 1998. Promoting notochord fate and repressing muscle development in zebrafish axial mesoderm. Development 125, 1397–1406. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
