This is a preprint.
Species Dependent Metabolism of a Covalent nsP2 Protease Inhibitor with in Vivo Anti-alphaviral Activity
- PMID: 39868137
- PMCID: PMC11761022
- DOI: 10.1101/2025.01.13.632788
Species Dependent Metabolism of a Covalent nsP2 Protease Inhibitor with in Vivo Anti-alphaviral Activity
Update in
-
Species-Dependent Metabolism of a Covalent nsP2 Protease Inhibitor with In Vivo Antialphaviral Activity.J Med Chem. 2025 May 22;68(10):10473-10485. doi: 10.1021/acs.jmedchem.5c00825. Epub 2025 May 12. J Med Chem. 2025. PMID: 40351160 Free PMC article.
Abstract
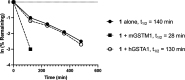
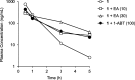
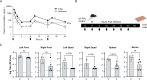
RA-0002034 (1) is a potent covalent inhibitor targeting the alphavirus nsP2 cysteine protease. The species-dependent pharmacokinetics and metabolism of 1 were investigated to evaluate its therapeutic potential. Pharmacokinetic profiling revealed rapid clearance in mice, predominantly mediated by glutathione S-transferase (GST)-catalyzed conjugation. This metabolic liability contrasted with slower clearance observed in human hepatocytes and preclinical species such as rats, dogs, and monkeys. Cross-species studies confirmed the dominance of GST-driven metabolism in mice, whereas oxidative pathways were more pronounced in dogs. Despite rapid systemic clearance, 1 achieved antiviral efficacy in mice, reducing CHIKV viral loads in multiple tissues. Initial estimations of human hepatic clearance and half-life extrapolated from animal data indicate that b.i.d. dosing of 1 will be possible to maintain concentrations sufficient for antiviral activity in humans. These cross-species pharmacokinetic and metabolism studies support the continued evaluation of 1 as a promising anti-alphaviral therapeutic.
Figures




Similar articles
-
Species-Dependent Metabolism of a Covalent nsP2 Protease Inhibitor with In Vivo Antialphaviral Activity.J Med Chem. 2025 May 22;68(10):10473-10485. doi: 10.1021/acs.jmedchem.5c00825. Epub 2025 May 12. J Med Chem. 2025. PMID: 40351160 Free PMC article.
-
Discovery of a cell-active chikungunya virus nsP2 protease inhibitor using a covalent fragment-based screening approach.bioRxiv [Preprint]. 2024 May 23:2024.03.22.586341. doi: 10.1101/2024.03.22.586341. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2024 Oct 15;121(42):e2409166121. doi: 10.1073/pnas.2409166121. PMID: 38562906 Free PMC article. Updated. Preprint.
-
Identification of a cell-active chikungunya virus nsP2 protease inhibitor using a covalent fragment-based screening approach.Proc Natl Acad Sci U S A. 2024 Oct 15;121(42):e2409166121. doi: 10.1073/pnas.2409166121. Epub 2024 Oct 10. Proc Natl Acad Sci U S A. 2024. PMID: 39388272 Free PMC article.
-
Recent advances in phytocompounds as potential Chikungunya virus non-structural protein 2 protease antagonists: A systematic review.Phytomedicine. 2025 Jan;136:156359. doi: 10.1016/j.phymed.2024.156359. Epub 2024 Dec 29. Phytomedicine. 2025. PMID: 39756312
-
Lopinavir/ritonavir: a review of its use in the management of HIV infection.Drugs. 2003;63(8):769-802. doi: 10.2165/00003495-200363080-00004. Drugs. 2003. PMID: 12662125 Review.
References
-
- Ghoshal A.; Asressu K. H.; Hossain M. A.; Brown P. J.; Nandakumar M.; Vala A.; Merten E. M.; Sears J. D.; Law I.; Burdick J. E.; et al. Structure Activity of beta-Amidomethyl Vinyl Sulfones as Covalent Inhibitors of Chikungunya nsP2 Cysteine Protease with Antialphavirus Activity. J Med Chem 2024, 67 (18), 16505–16532. DOI: 10.1021/acs.jmedchem.4c01346 - DOI - PMC - PubMed
-
- Merten E. M.; Sears J. D.; Leisner T. M.; Hardy P. B.; Ghoshal A.; Hossain M. A.; Asressu K. H.; Brown P. J.; Tse E. G.; Stashko M. A.; et al. Identification of a cell-active chikungunya virus nsP2 protease inhibitor using a covalent fragment-based screening approach. Proc Natl Acad Sci U S A 2024, 121 (42), e2409166121. DOI: 10.1073/pnas.2409166121 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
