This is a preprint.
Terminal complement complexes with or without C9 potentiate antimicrobial activity against Neisseria gonorrhoeae
- PMID: 39868146
- PMCID: PMC11760736
- DOI: 10.1101/2025.01.16.633325
Terminal complement complexes with or without C9 potentiate antimicrobial activity against Neisseria gonorrhoeae
Update in
-
Terminal complement complexes with or without C9 potentiate antimicrobial activity against Neisseria gonorrhoeae.mBio. 2025 May 14;16(5):e0014125. doi: 10.1128/mbio.00141-25. Epub 2025 Mar 31. mBio. 2025. PMID: 40162779 Free PMC article.
Abstract
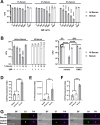
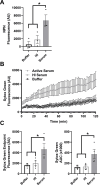
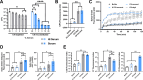
The complement cascade is a front-line defense against pathogens. Complement activation generates the membrane attack complex (MAC), a 10-11 nm diameter pore formed by complement proteins C5b through C8 and polymerized C9. The MAC embeds within the outer membrane of Gram-negative bacteria and displays bactericidal activity. In the absence of C9, C5b-C8 complexes can form 2-4 nm pores on membranes, but their relevance to microbial control is poorly understood. Deficiencies in terminal complement components uniquely predispose individuals to infections by pathogenic Neisseria, including N. gonorrhoeae (Gc). Increasing antibiotic resistance in Gc makes new therapeutic strategies a priority. Here, we demonstrate that MAC formed by complement activity in human serum disrupts the Gc outer and inner membranes, potentiating the activity of antimicrobials against Gc and re-sensitizing multidrug resistant Gc to antibiotics. C9-depleted serum also disrupts Gc membranes and exerts antigonococcal activity, effects that are not reported in other Gram-negative bacteria. C5b-C8 complex formation potentiates Gc sensitivity to azithromycin but not lysozyme. These findings expand our mechanistic understanding of complement lytic activity, suggest a size limitation for terminal complement-mediated enhancement of antimicrobials against Gc, and suggest complement manipulation can be used to combat drug-resistant gonorrhea.
Importance: The complement cascade is a front-line arm of the innate immune system against pathogens. Complement activation results in membrane attack complex (MAC) pores forming on the outer membrane of Gram-negative bacteria, resulting in bacterial death. Individuals who cannot generate MAC are specifically susceptible to infection by pathogenic Neisseria species including N. gonorrhoeae (Gc). High rates of gonorrhea and its complications like infertility, and high-frequency resistance to multiple antibiotics, make it important to identify new approaches to combat Gc. Beyond direct anti-Gc activity, we found the MAC increases the ability of antibiotics and antimicrobial proteins to kill Gc and re-sensitizes multidrug-resistant bacteria to antibiotics. The most terminal component, C9, is needed to potentiate the anti-Gc activity of lysozyme, but azithromycin activity is potentiated regardless of C9. These findings highlight the unique effects of MAC on Gc and suggest novel translational avenues to combat drug-resistant gonorrhea.
Keywords: Antimicrobial resistance; Complement; Innate immunity; Membrane attack complex; Neisseria; Neisseria gonorrhoeae.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures




Similar articles
-
Terminal complement complexes with or without C9 potentiate antimicrobial activity against Neisseria gonorrhoeae.mBio. 2025 May 14;16(5):e0014125. doi: 10.1128/mbio.00141-25. Epub 2025 Mar 31. mBio. 2025. PMID: 40162779 Free PMC article.
-
Polymerization of C9 enhances bacterial cell envelope damage and killing by membrane attack complex pores.PLoS Pathog. 2021 Nov 9;17(11):e1010051. doi: 10.1371/journal.ppat.1010051. eCollection 2021 Nov. PLoS Pathog. 2021. PMID: 34752492 Free PMC article.
-
Serum Complement Activation by C4BP-IgM Fusion Protein Can Restore Susceptibility to Antibiotics in Neisseria gonorrhoeae.Front Immunol. 2021 Sep 1;12:726801. doi: 10.3389/fimmu.2021.726801. eCollection 2021. Front Immunol. 2021. PMID: 34539665 Free PMC article.
-
The killer molecule of complement.J Invest Dermatol. 1985 Jul;85(1 Suppl):47s-52s. doi: 10.1111/1523-1747.ep12275445. J Invest Dermatol. 1985. PMID: 3891882 Review.
-
Structural biology of the membrane attack complex.Subcell Biochem. 2014;80:83-116. doi: 10.1007/978-94-017-8881-6_6. Subcell Biochem. 2014. PMID: 24798009 Review.
References
-
- Lewis LA, Ram S. 2020. Complement interactions with the pathogenic Neisseriae: clinical features, deficiency states, and evasion mechanisms. FEBS Letters 594:2670–2694. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
