This is a preprint.
Oral prodrug of a novel glutathione surrogate reverses metabolic dysregulation and attenuates neurodegenerative process in APP/PS1 mice
- PMID: 39868172
- PMCID: PMC11761491
- DOI: 10.1101/2025.01.15.633247
Oral prodrug of a novel glutathione surrogate reverses metabolic dysregulation and attenuates neurodegenerative process in APP/PS1 mice
Update in
-
Oral Prodrug of a Novel Glutathione Surrogate Reverses Metabolic Dysregulation and Attenuates Neurodegenerative Process in Transgenic Alzheimer's Mice.ACS Pharmacol Transl Sci. 2025 Jul 18:10.1021/acsptsci.5c00031. doi: 10.1021/acsptsci.5c00031. Online ahead of print. ACS Pharmacol Transl Sci. 2025. PMID: 40726727 Free PMC article.
Abstract
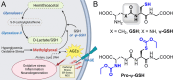
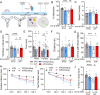
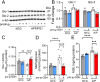
Glycation-induced oxidative stress underlies the numerous metabolic ravages of Alzheimer's disease (AD). Reduced glutathione levels in AD lead to increased oxidative stress, including glycation-induced pathology. Previously, we showed that the accumulation of reactive 1,2-dicarbonyls such as methylglyoxal, the major precursor of non-enzymatic glycation products, was reduced by the increased function of GSH-dependent glyoxalase-1 enzyme in the brain. In this two-pronged study, we evaluate the therapeutic efficacy of an orally bioavailable prodrug of our lead glyoxalase substrate, pro-ψ-GSH, for the first time in a transgenic Alzheimer's disease mouse model. This prodrug delivers pharmacodynamically relevant brain concentrations of ψ-GSH upon oral delivery. Chronic oral dosing of pro-ψ-GSH effectively reverses the cognitive decline observed in the APP/PS1 mouse model. The prodrug successfully mirrors the robust effects of the parent drug i.e., reducing amyloid pathology, glycation stress, neuroinflammation, and the resultant neurodegeneration in these mice. We also report the first metabolomics study of such a treatment, which yields key biomarkers linked to the reversal of AD-related metabolic dysregulation. Collectively, this study establishes pro-ψ-GSH as a viable, disease-modifying therapy for AD and paves the way for further preclinical advancement of such therapeutics. Metabolomic signatures identified could prove beneficial in the development of treatment-specific clinically translatable biomarkers.
Keywords: Alzheimer’s disease; Glyoxalase-1; advanced glycation end products; metabolomics; neuroinflammation; oxidative stress.
Conflict of interest statement
Competing interests S.S.M. and R.V. are co-inventors on the patent applications relating to ψ-GSH and its analogs as treatment options for neurodegenerative disorders and liver diseases. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures






Similar articles
-
Oral Prodrug of a Novel Glutathione Surrogate Reverses Metabolic Dysregulation and Attenuates Neurodegenerative Process in Transgenic Alzheimer's Mice.ACS Pharmacol Transl Sci. 2025 Jul 18:10.1021/acsptsci.5c00031. doi: 10.1021/acsptsci.5c00031. Online ahead of print. ACS Pharmacol Transl Sci. 2025. PMID: 40726727 Free PMC article.
-
Intranasal Delivery of Metabolically Resilient Glutathione: In Vivo Pharmacokinetic, Permeation, and Efficacy Studies.Mol Pharm. 2025 Jul 7;22(7):4145-4157. doi: 10.1021/acs.molpharmaceut.5c00382. Epub 2025 May 22. Mol Pharm. 2025. PMID: 40401709
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Methylglyoxal-induced glycation stress promotes aortic stiffening: Putative mechanistic roles of oxidative stress and cellular senescence.bioRxiv [Preprint]. 2025 Jan 6:2025.01.06.631561. doi: 10.1101/2025.01.06.631561. bioRxiv. 2025. PMID: 39829921 Free PMC article. Preprint.
-
A systematic review of the efficacy and safety of anti-amyloid beta monoclonal antibodies in treatment of Alzheimer's disease.Expert Opin Biol Ther. 2024 Nov;24(11):1261-1269. doi: 10.1080/14712598.2024.2416947. Epub 2024 Nov 12. Expert Opin Biol Ther. 2024. PMID: 39432414
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
