This is a preprint.
Insights into the Absence of Lymphoma Despite Fulminant Epstein-Barr Virus Infection in Patients with XIAP Deficiency
- PMID: 39868266
- PMCID: PMC11761029
- DOI: 10.1101/2025.01.17.633616
Insights into the Absence of Lymphoma Despite Fulminant Epstein-Barr Virus Infection in Patients with XIAP Deficiency
Update in
-
Insights into absence of lymphoma despite fulminant Epstein-Barr virus infection in patients with XIAP deficiency.JCI Insight. 2025 Jul 15;10(16):e193787. doi: 10.1172/jci.insight.193787. eCollection 2025 Aug 22. JCI Insight. 2025. PMID: 40674368 Free PMC article.
Abstract
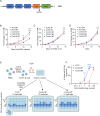
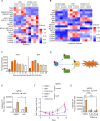
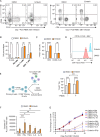
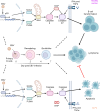
X-linked Lymphoproliferative Syndromes (XLP), which arise from mutations in the SH2D1A or XIAP genes, are characterized by the inability to control Epstein-Barr Virus (EBV) infection. While primary EBV infection triggers severe diseases in each, lymphomas occur at high rates with XLP-1 but not with XLP-2. Why XLP-2 patients are apparently protected from EBV-driven lymphomagenesis, in contrast to all other described congenital conditions that result in heightened susceptibility to EBV, remains a key open question. To gain insights, we cross-compared newly EBV infected versus immune stimulated B-cells from XLP-2 patients or upon XIAP CRISPR knockout, relative to healthy controls. XIAP perturbation impeded outgrowth of newly EBV-infected primary human B-cells, though had no impact on proliferation of B-cells stimulated by CD40 ligand and interleukin-21 or upon established EBV-immortalized lymphoblastoid cell lines (LCLs). B-cells from XLP-2 patients or in which XIAP was depleted by CRISPR editing exhibited a markedly lower EBV transformation efficiency than healthy control B-cells. Mechanistically, nascent EBV infection activated p53-mediated apoptosis signaling, whose effects on transforming B-cell death were counteracted by XIAP. In the absence of XIAP, EBV infection triggered high rates of apoptosis, not seen with CD40L/IL-21 stimulation. Moreover, inflammatory cytokines are present at high levels in XLP-2 patient serum with fulminant EBV infection, which heightened apoptosis induction in newly EBV-infected cells. These findings highlight the crucial role of XIAP in supporting early stages of EBV-driven B-cell immortalization and provide insights into the absence of EBV+ lymphoma in XLP-2 patients.
Conflict of interest statement
Conflict-of-interest disclosure The authors declare no competing financial interests.
Figures


References
-
- Farrell PJ. Epstein–Barr Virus and Cancer. Annual Review of Pathology: Mechanisms of Disease. 2019;14(Volume 14, 2019):29–53. - PubMed
-
- Latency Münz C. and lytic replication in Epstein–Barr virus-associated oncogenesis. Nature Reviews Microbiology. 2019;17(11):691–700. - PubMed
-
- Tangye SG, Latour S. Primary immunodeficiencies reveal the molecular requirements for effective host defense against EBV infection. Blood. 2020;135(9):644–655. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
