This is a preprint.
Initiation of primary T cell-B cell interactions and extrafollicular antibody responses in an organized microphysiological model of the human lymph node
- PMID: 39868310
- PMCID: PMC11761657
- DOI: 10.1101/2025.01.12.632545
Initiation of primary T cell-B cell interactions and extrafollicular antibody responses in an organized microphysiological model of the human lymph node
Abstract
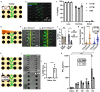
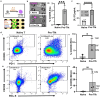
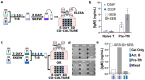
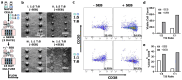
Antibody production is central to protection against new pathogens and cancers, as well as to certain forms of autoimmunity. Antibodies often originate in the lymph node (LN), specifically at the extrafollicular border of B cell follicles, where T and B lymphocytes physically interact to drive B cell maturation into antibody-secreting plasmablasts. In vitro models of this process are sorely needed to predict aspects of the human immune response. Microphysiological systems (MPSs) offer the opportunity to approximate the lymphoid environment, but so far have focused primarily on memory recall responses to antigens previously encountered by donor cells. To date, no 3D culture system has replicated the engagement between T cells and B cells (T-B interaction) that leads to antibody production when starting with naïve cells. Here, we developed a LN-MPS to model early T-B interactions at the extrafollicular border built from primary, naïve human lymphocytes encapsulated within a collagen-based 3D matrix. Within the MPS, naïve T cells exhibited CCL21-dependent chemotaxis and chemokinesis as predicted. Naïve T and B cells were successfully skewed on chip to an early T follicular helper (pre-Tfh) and activated state, respectively, and co-culture of the latter cells led to CD38+ plasmablast cells and T cell dependent production of IgM. These responses required differentiation of the T cells into pre-Tfhs, physical cell-cell contact, and were sensitive to the ratio at which pre-Tfh and activated B cells were seeded on-chip. Dependence on T cell engagement was greatest at a 1:5 T:B ratio, while cell proliferation and CD38+ signal was greatest at a 1:1 T:B ratio. Furthermore, plasmablast formation was established starting from naïve T and B cells on-chip. We envision that this MPS model of primary lymphocyte physiology will enable new mechanistic analyses of human humoral immunity in vitro.
Conflict of interest statement
DECLARATION OF COMPETING INTEREST JOC and RRP are listed as inventors on a patent application (Serial No. 17/045,459) filed by the University of Virginia related to spatially patterned lymph node organ-on-chip systems. All other authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Murphy K.; Weaver C. Janeway’s Immunobiology; Garland Science, 2016.
-
- Sathe A.; Cusick J. K. Biochemistry, Immunoglobulin M. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2025. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
