Breast Cancer-Derived Extracellular Vesicles Modulate the Cytoplasmic and Cytoskeletal Dynamics of Blood-Brain Barrier Endothelial Cells
- PMID: 39868462
- PMCID: PMC11770372
- DOI: 10.1002/jev2.70038
Breast Cancer-Derived Extracellular Vesicles Modulate the Cytoplasmic and Cytoskeletal Dynamics of Blood-Brain Barrier Endothelial Cells
Abstract
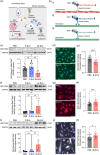
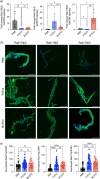
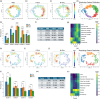
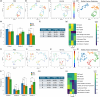
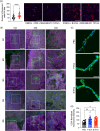
Extracellular vesicles (EVs) from brain-seeking breast cancer cells (Br-EVs) breach the blood-brain barrier (BBB) via transcytosis and promote brain metastasis. Here, we defined the mechanisms by which Br-EVs modulate brain endothelial cell (BEC) dynamics to facilitate their BBB transcytosis. BEC treated with Br-EVs show significant downregulation of Rab11fip2, known to promote vesicle recycling to the plasma membrane and significant upregulation of Rab11fip3 and Rab11fip5, which support structural stability of the endosomal compartment and facilitate vesicle recycling and transcytosis, respectively. Using machine learning and quantitative global proteomic, we identified novel Br-EV-induced changes in BECs morphology, motility, and proteome that correlate with decreased BEC cytoplasm and cytoskeletal organization and dynamics. These results define early steps leading to breast-to-brain metastasis and identify molecules that could serve as targets for therapeutic strategies for brain metastasis.
Keywords: blood‐brain barrier; brain metastasis; breast cancer; exosomes; extracellular vesicles; microvesicles; pre‐metastatic niche.
© 2025 The Author(s). Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Andrew, Y. , Ng M. I. J., Weiss Y.. 2001. On Spectral Clustering: Analysis and an Algorithm. Cambridge, MA: MIT Press.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

