VAX014 Activates Tumor-Intrinsic STING and RIG-I to Promote the Development of Antitumor Immunity
- PMID: 39868467
- PMCID: PMC11962400
- DOI: 10.1158/1535-7163.MCT-24-0509
VAX014 Activates Tumor-Intrinsic STING and RIG-I to Promote the Development of Antitumor Immunity
Abstract
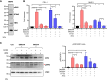
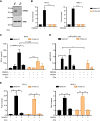
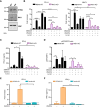
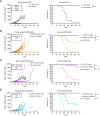
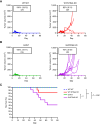
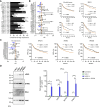
In situ immunization (ISI) has emerged as a promising approach to bolster early phases of the cancer immunity cycle through improved T-cell priming. One class of ISI agents, oncolytic viruses (OV), has demonstrated clinical activity, but overall benefit remains limited. Mounting evidence suggests that due to their inherent vulnerability to antiviral effects of type I IFN, OVs have limited activity in solid tumors expressing stimulator of interferon genes (STING) and/or retinoic acid-inducible gene I (RIG-I). Here, using a combination of pharmacologic, genetic, and in vivo approaches, we demonstrate that VAX014, a bacterial minicell-based oncolytic ISI agent, activates both STING and RIG-I and leverages this activity to work best in STING-positive and/or RIG-I-positive tumors. Intratumoral treatment of established syngeneic tumors expressing STING and RIG-I with VAX014 resulted in 100% tumor clearance in two mouse models. Antitumor activity of VAX014 was shown to be dependent on both tumor-intrinsic STING and RIG-I with additive activity stemming from host-intrinsic STING. Analysis of human solid tumor datasets demonstrated STING and RIG-I co-expression is prevalent in solid tumors and associates with clinical benefit in many indications, particularly those most amenable to intratumoral administration. These collective findings differentiate VAX014 from OVs by elucidating the ability of this agent to elicit antitumor activity in STING-positive and/or RIG-I-positive solid tumors and provide evidence that STING/RIG-I agonism is part of VAX014's mechanism of action. Taken together, this work supports the ongoing clinical investigation of VAX014 treatment as an alternative to OV therapy in patients with solid tumors.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
C.D. House reports grants from Vaxiion Therapeutics during the conduct of the study, as well as grants from the NCI and the Conrad Prebys Foundation outside the submitted work. K.L. McGuire reports grants from Vaxiion Therapeutics during the conduct of the study. M.J. Giacalone reports a patent for PCT/US2024/011105 pending. No disclosures were reported by the other authors.
Figures

Similar articles
-
Intravesical VAX014 Synergizes with PD-L1 Blockade to Enhance Local and Systemic Control of Bladder Cancer.Cancer Immunol Res. 2022 Aug 3;10(8):978-995. doi: 10.1158/2326-6066.CIR-21-0879. Cancer Immunol Res. 2022. PMID: 35679299 Free PMC article.
-
STING activator 2'3'-cGAMP enhanced HSV-1-based oncolytic viral therapy.Mol Oncol. 2024 May;18(5):1259-1277. doi: 10.1002/1878-0261.13603. Epub 2024 Feb 23. Mol Oncol. 2024. PMID: 38400597 Free PMC article.
-
Intralesional administration of VAX014 facilitates in situ immunization and potentiates immune checkpoint blockade in immunologically cold tumors.J Immunother Cancer. 2023 Jun;11(6):e006749. doi: 10.1136/jitc-2023-006749. J Immunother Cancer. 2023. PMID: 37290924 Free PMC article.
-
Oncolytic Viruses: Priming Time for Cancer Immunotherapy.BioDrugs. 2019 Oct;33(5):485-501. doi: 10.1007/s40259-019-00367-0. BioDrugs. 2019. PMID: 31321623 Free PMC article. Review.
-
Antitumor Benefits of Antiviral Immunity: An Underappreciated Aspect of Oncolytic Virotherapies.Trends Immunol. 2018 Mar;39(3):209-221. doi: 10.1016/j.it.2017.11.006. Epub 2017 Dec 20. Trends Immunol. 2018. PMID: 29275092 Review.
Cited by
-
Antiviral nanomedicine: Advantages, mechanisms and advanced therapies.Bioact Mater. 2025 Jun 5;52:92-122. doi: 10.1016/j.bioactmat.2025.05.030. eCollection 2025 Oct. Bioact Mater. 2025. PMID: 40530413 Free PMC article. Review.
References
-
- Morad G, Helmink BA, Sharma P, Wargo JA. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 2022;185:576. - PubMed
-
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013;39:1–10. - PubMed
-
- Mellman I, Chen DS, Powles T, Turley SJ. The cancer-immunity cycle: indication, genotype, and immunotype. Immunity 2023;56:2188–205. - PubMed
-
- Budimir N, Thomas GD, Dolina JS, Salek-Ardakani S. Reversing T-cell exhaustion in cancer: lessons learned from PD-1/PD-L1 immune checkpoint blockade. Cancer Immunol Res 2022;10:146–53. - PubMed
-
- O'Donnell JS, Long GV, Scolyer RA, Teng MWL, Smyth MJ. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat Rev 2017;52:71–81. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

