Association of rapid eye movement sleep latency with multimodal biomarkers of Alzheimer's disease
- PMID: 39868572
- PMCID: PMC11848184
- DOI: 10.1002/alz.14495
Association of rapid eye movement sleep latency with multimodal biomarkers of Alzheimer's disease
Abstract
Introduction: Sleep disturbances are associated with Alzheimer's disease (AD) and Alzheimer's disease and related dementias (ADRD), but the relationship between sleep architecture, particularly rapid eye movement (REM) sleep, and AD/ADRD biomarkers remains unclear.
Methods: We enrolled 128 adults (64 with Alzheimer's disease, 41 with mild cognitive impairment [MCI], and 23 with normal cognition [NC]), mean age 70.8 ± 9.6 years, 56.9% female, from a tertiary hospital in China. Participants underwent overnight polysomnography (PSG), amyloid β (Aβ) positron emission tomography (PET), and plasma biomarker analysis: phosphorylated tau at threonine 181 (p-tau181), neurofilament light (NfL), and brain-derived neurotrophic factor (BDNF).
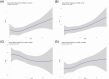
Results: After adjusting for demographics, apolipoprotein E (APOE) ε4 status, cognition, and comorbidities, the highest tertile of REM latency was associated with higher Aβ burden (β = 0.08, 95% confidence interval [CI]: 0.03 to 0.13, p = 0.002), elevated p-tau181 (β = 0.19, 95% CI: 0.02 to 0.13, p = 0.002), and reduced BDNF levels (β = -0.47, 95% CI: -0.68 to -0.13, p = 0.013), compared to the lowest tertile.
Discussion: Prolonged REM latency may serve as a novel marker or risk factor for AD/ADRD pathogenesis.
Highlights: Rapid eye movement latency (REML) may be a potential marker for Alzheimer's disease and Alzheimer's disease and related dementias (AD/ADRD) pathogenesis. Prolonged REML was associated with higher amyloid beta (Aβ) burden, phosphorylated tau-181 (p-tau181), and lower brain-derived neurotrophic factor (BDNF) levels. Intervention trial is needed to determine if targeting REML can modify AD/ADRD risk. Slow-wave sleep was not associated with AD/ADRD biomarkers.
Keywords: Alzheimer's disease; amyloid beta; biomarker; polysomnography; rapid eye movement latency; sleep.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Dr. Lucey discloses funding from the National Institutes of Health, Eisai, and Good Ventures/Open Philanthropy that are outside the scope of this work. Dr. Lucey also discloses consulting for Merck, Eli Lilly, Eisai, OrbiMed, and GLG Consulting, as well as honoraria from the BrightFocus Foundation, the Weston Brain Institute, Eli Lilly, and Beacon Biosignals that are outside the scope of this work. All other authors declare not competing interests. Author disclosures are available in the Supporting Information.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

