Postprandial time in tight range with faster insulin aspart compared with standard insulin aspart in youth with type 1 diabetes using automated insulin delivery
- PMID: 39868600
- PMCID: PMC11885092
- DOI: 10.1111/dom.16211
Postprandial time in tight range with faster insulin aspart compared with standard insulin aspart in youth with type 1 diabetes using automated insulin delivery
Abstract
Aims: The aim of this study was to assess postprandial glycaemic outcomes using automated insulin delivery with faster acting insulin aspart (FIA) or standard insulin aspart (SIA) over 4 weeks in youth (aged 10-18 years) with type 1 diabetes.
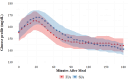
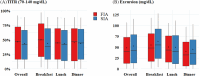
Materials and methods: We undertook a secondary analysis of postprandial glycaemic outcomes from a double-blind, randomised, crossover study comparing FIA to SIA using an investigational version of MiniMed™ 780G. Endpoints included postprandial time in tight range (70-140 mg/dL; TITR), postprandial glucose excursions and peak glucose, and incremental area under curve (iAUC).
Results: The mean ± SD age of 30 included participants was 15.0 ± 1.7 years, 47% were male, mean HbA1c was 7.5% ± 0.9% (58 ± 9.8 mmol/mol) and the number of meals per day per participant was 3.2 ± 1.2 meals. Overall, the postprandial outcomes were improved with FIA compared with SIA. Mean glucose at the start of the meal was 151 mg/dL in the FIA group and reached a peak glucose of 194 mg/dL, compared with starting level of 151 mg/dL in the SIA group and a peak of 198 mg/dL (difference in excursion: -3.8 mg/dL; 95% confidence interval -5.8 to -1.7; p <0.001). FIA group also had a 1.9% increase in mean TITR (p = 0.02) and a 2.0-mg/dL decrease in mean iAUC (p = 0.003). Differences in outcomes were the most noticeable for breakfast, meals with a larger amount of carbohydrates (>45 g) and participants with lower insulin-to-carbohydrate ratios.
Conclusions: Faster insulin formulation with AID improved postprandial glycaemic outcomes and could be a useful therapeutical option in youth with type 1 diabetes that have challenges achieving glycaemic targets.
Keywords: continuous glucose monitoring (CGM); glycaemic control; insulin therapy; type 1 diabetes.
© 2025 The Author(s). Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
KD has received honoraria for speaking engagements from Abbott, Eli Lilly, Medtronic, Novo Nordisk and Pfizer and is on an advisory board for Medtronic and Novo Nordisk. EFR has received honoraria for speaking engagements and advisory boards from Medtronic, Eli Lilly, Sanofi, Merck and Sandoz. DPZ has received honoraria for speaking engagements from Ascensia Diabetes, Insulet Canada, Dexcom Canada and Medtronic and is on an advisory board for Dexcom and has received an ISPAD‐JDRF Research Fellowship and research support from Insulet and the Leona M, and Harry B. Helmsley Charitable Trust. TB received honoraria for participating on the speaker's bureaux of Eli Lilly, Novo Nordisk, Medtronic, Abbott, Sanofi, Dexcom, Aventis, Astra Zeneca and Roche. TB's institution received research grant support from Abbott, Medtronic, Novo Nordisk, Sanofi, Novartis, Sandoz, Zealand Pharma, Slovenian Research Agency, the National Institutes of Health and the European Union. Other authors report no conflicts of interest.
Figures
References
-
- Sherr JL, Schoelwer M, Dos Santos TJ, et al. ISPAD clinical practice consensus guidelines 2022: diabetes technologies: insulin delivery. Pediatr Diabetes. 2022;23(8):1406‐1431. - PubMed
-
- Stamati A, Karagiannis T, Tsapas A, Christoforidis A. Efficacy and safety of ultra‐rapid insulin analogues in insulin pumps in patients with type 1 diabetes mellitus: a systematic review and meta‐analysis. Diabetes Res Clin Pract. 2022;193:110144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

