Fluorofurimazine, a novel NanoLuc substrate, enhances real-time tracking of influenza A virus infection without altering pathogenicity in mice
- PMID: 39868868
- PMCID: PMC11878008
- DOI: 10.1128/spectrum.02689-24
Fluorofurimazine, a novel NanoLuc substrate, enhances real-time tracking of influenza A virus infection without altering pathogenicity in mice
Abstract
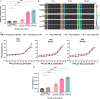
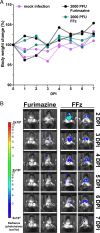
Bioluminescence imaging (BLI) using engineered bioluminescent viruses has emerged as a powerful tool for real-time, noninvasive monitoring of viral replication in living animals. While traditional luciferase-based systems, such as firefly luciferase, have been widely used, the NanoLuc luciferase system offers distinct advantages, including its significantly smaller gene size, increased brightness, and independence from ATP as a cofactor, allowing for extracellular detection. However, the utility of NanoLuc has been limited by its traditional substrate, furimazine, which exhibits poor water solubility and potential cytotoxicity. In this study, we assessed fluorofurimazine (FFz), a novel substrate with improved water solubility and bioavailability, for tracking influenza A virus (IAV) replication in mice. Our findings demonstrate that FFz substantially enhances detection sensitivity in both respiratory organs and brain tissue without increasing toxicity, enabling more precise and sustained monitoring of IAV replication. In vitro, FFz generated higher photon flux at lower concentrations compared to furimazine, translating into superior in vivo sensitivity with reduced toxicity. Crucially, FFz did not alter the pathogenicity of IAV in mice, even at sublethal infectious doses, reinforcing its suitability for use in BLI-based viral pathogenicity studies. These results suggest that combining FFz with NanoLuc provides a more effective and less toxic approach for real-time tracking of viral infections in preclinical models.
Importance: Monitoring viral infections in living animals is a valuable approach for understanding how viruses replicate and cause disease. This study focuses on bioluminescent influenza A virus infection in a mouse model and evaluates fluorofurimazine, a new substrate that enhances bioluminescence imaging. Fluorofurimazine allows researchers to monitor viral spread more effectively than the traditional substrate, furimazine, which is often toxic and less reliable. It offers better sensitivity and lower toxicity, enabling longer and more accurate tracking of viral replication in the lungs and even the brain. Importantly, fluorofurimazine does not alter the pathogenicity of the virus, providing an unaltered representation of the infection process. This advancement has the potential to significantly improve how scientists study bioluminescent viral infections and evaluate antiviral drugs and vaccines, making it a valuable tool for research on influenza and other respiratory viruses.
Keywords: NanoLuc; bioluminescence; brain; fluorofurimazine; furimazine; influenza A virus; respiratory system; toxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

