Nanoparticle-Mediated Cuproptosis and Photodynamic Synergistic Strategy: A Novel Horizon for Cancer Therapy
- PMID: 39868904
- PMCID: PMC11770888
- DOI: 10.1002/cam4.70599
Nanoparticle-Mediated Cuproptosis and Photodynamic Synergistic Strategy: A Novel Horizon for Cancer Therapy
Erratum in
-
Correction to "Nanoparticle-Mediated Cuproptosis and Photodynamic Synergistic Strategy: A Novel Horizon for Cancer Therapy".Cancer Med. 2025 Jul;14(14):e71022. doi: 10.1002/cam4.71022. Cancer Med. 2025. PMID: 40702718 Free PMC article. No abstract available.
Abstract
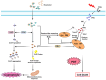
Background: Photodynamic therapy (PDT) is a noninvasive cancer treatment that works by using light to stimulate the production of excessive cytotoxic reactive oxygen species (ROS), which effectively eliminates tumor cells. However, the therapeutic effects of PDT are often limited by tumor hypoxia, which prevents effective tumor cell elimination. The oxygen (O2) consumption during PDT can further exacerbate hypoxia, leading to post-treatment adverse events.
Objectives: This review aims to explore the potential of cuproptosis, a recently discovered copper-dependent form of programmed cell death, to enhance the anticancer effects of PDT. Cuproptosis is highly dependent on mitochondrial respiration, specifically the tricarboxylic acid (TCA) cycle, and can increase O2 and ROS levels or decrease glutathione (GSH) levels, thereby improving PDT outcomes.
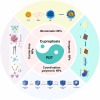
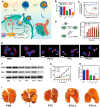
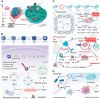
Methods: The review discusses the latest research advancements in the field, detailing the mechanisms that regulate cuproptosis and PDT. It also explores how nanoparticle (NP)-based strategies can be used to exploit the synergistic potential between cuproptosis and PDT. The article examines the prospects of synergistic anticancer activity guided by nanodelivery systems, which could overcome the challenges associated with hypoxia in cancer treatment.
Conclusions: The combination of cuproptosis and PDT, facilitated by NP-based delivery systems, presents a promising approach to enhance the effectiveness of cancer therapy. The review concludes by discussing the challenges and future research directions for this combination therapy, highlighting the need for further investigation into the mechanisms and optimization of treatment strategies to improve outcomes in cancer treatment.
Keywords: PDT; cancer; cuproptosis; therapy nanomaterials.
© 2025 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Synergistic cancer treatment with PCuLu nanocatalysts by inducing cuproptosis and enhancing radiotherapy.J Colloid Interface Sci. 2025 Dec;699(Pt 2):138260. doi: 10.1016/j.jcis.2025.138260. Epub 2025 Jun 20. J Colloid Interface Sci. 2025. PMID: 40578089
-
The safety and efficiency of photodynamic therapy for the treatment of osteosarcoma: A systematic review of in vitro experiment and animal model reports.Photodiagnosis Photodyn Ther. 2022 Dec;40:103093. doi: 10.1016/j.pdpdt.2022.103093. Epub 2022 Aug 27. Photodiagnosis Photodyn Ther. 2022. PMID: 36031143
-
Cuproptosis: mechanisms and nanotherapeutic strategies in cancer and beyond.Chem Soc Rev. 2025 Jun 30;54(13):6282-6334. doi: 10.1039/d5cs00083a. Chem Soc Rev. 2025. PMID: 40433941 Review.
-
A systematic review of photodynamic therapy in the treatment of pre-cancerous skin conditions, Barrett's oesophagus and cancers of the biliary tract, brain, head and neck, lung, oesophagus and skin.Health Technol Assess. 2010 Jul;14(37):1-288. doi: 10.3310/hta14370. Health Technol Assess. 2010. PMID: 20663420
-
Overcoming resistant cancerous tumors through combined photodynamic and immunotherapy (photoimmunotherapy).Front Immunol. 2025 Jul 17;16:1633953. doi: 10.3389/fimmu.2025.1633953. eCollection 2025. Front Immunol. 2025. PMID: 40746528 Free PMC article. Review.
Cited by
-
Targeting cuproptosis opens a new chapter of nanomedicine: a scientometric and graphical analysis.Naunyn Schmiedebergs Arch Pharmacol. 2025 Aug 12. doi: 10.1007/s00210-025-04497-x. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40794178
-
Photodynamic therapeutic activity of novel porphyrins against lung squamous cell carcinoma.BMC Cancer. 2025 May 28;25(1):960. doi: 10.1186/s12885-025-14386-4. BMC Cancer. 2025. PMID: 40437394 Free PMC article.
-
Integrating cuproptosis and immunosenescence: A novel therapeutic strategy in cancer treatment.Biochem Biophys Rep. 2025 Mar 27;42:101983. doi: 10.1016/j.bbrep.2025.101983. eCollection 2025 Jun. Biochem Biophys Rep. 2025. PMID: 40224540 Free PMC article. Review.
References
-
- Bray F., Laversanne M., Sung H., et al., “Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries,” CA: A Cancer Journal for Clinicians 74, no. 3 (2024): 229–263. - PubMed
-
- Kit O. I., Katelnitskaya O. V., Maslov A. A., et al., “Combined Surgeries for Retroperitoneal Tumors,” Journal of Clinical Oncology 38, no. 15_suppl (2020): e23579.
-
- D'alterio C., Scala S., Sozzi G., et al., “Paradoxical Effects of Chemotherapy on Tumor Relapse and Metastasis Promotion,” Seminars in Cancer Biology 60 (2020): 351–361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

