Modeling the effects of thin filament near-neighbor cooperative interactions in mammalian myocardium
- PMID: 39869069
- PMCID: PMC11771317
- DOI: 10.1085/jgp.202413582
Modeling the effects of thin filament near-neighbor cooperative interactions in mammalian myocardium
Abstract
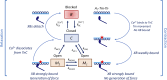
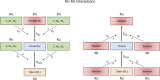
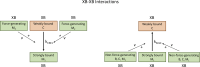
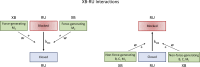
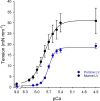
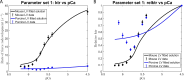
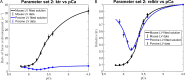
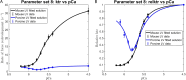
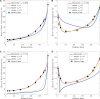
The mechanisms underlying cooperative activation and inactivation of myocardial force extend from local, near-neighbor interactions involving troponin-tropomyosin regulatory units (RU) and crossbridges (XB) to more global interactions across the sarcomere. To better understand these mechanisms in the hearts of small and large mammals, we undertook a simplified mathematical approach to assess the contribution of three types of near-neighbor cooperative interactions, i.e., RU-induced, RU-activation (RU-RU), crossbridge-induced, crossbridge-binding (XB-XB), and XB-induced, RU-activation (XB-RU). We measured the Ca2+ and activation dependence of the rate constant of force redevelopment in murine- and porcine-permeabilized ventricular myocardium. Mathematical modeling of these three near-neighbor interactions yielded nonlinear expressions for the RU-RU and XB-RU rate coefficients (kon and koff) and XB-XB rate coefficients describing the attachment of force-generating crossbridges (f and f'). The derivation of single cooperative coefficient parameters (u = RU-RU, w = XB-RU, and v = XB-XB) permitted an initial assessment of the strength of each near-neighbor interaction. The parameter sets describing the effects of discrete XB-XB or XB-RU interactions failed to adequately fit the in vitro contractility data in either murine or porcine myocardium. However, the Ca2+ dependence of ktr in murine and porcine ventricular myocardium was well fit by parameter sets incorporating the RU-RU cooperative interaction. Our results indicate that a significantly stronger RU-RU interaction is present in porcine ventricular myocardium compared with murine ventricular myocardium and that the relative strength of the near-neighbor RU-RU interaction contributes to species-specific myocardial contractile dynamics in small and large mammals.
© 2025 Phan and Fitzsimons.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures

Similar articles
-
Filament compliance influences cooperative activation of thin filaments and the dynamics of force production in skeletal muscle.PLoS Comput Biol. 2012;8(5):e1002506. doi: 10.1371/journal.pcbi.1002506. Epub 2012 May 10. PLoS Comput Biol. 2012. PMID: 22589710 Free PMC article.
-
A Spatially Detailed Model of Isometric Contraction Based on Competitive Binding of Troponin I Explains Cooperative Interactions between Tropomyosin and Crossbridges.PLoS Comput Biol. 2015 Aug 11;11(8):e1004376. doi: 10.1371/journal.pcbi.1004376. eCollection 2015 Aug. PLoS Comput Biol. 2015. PMID: 26262582 Free PMC article.
-
Cooperative mechanisms underlie differences in myocardial contractile dynamics between large and small mammals.J Gen Physiol. 2023 Nov 6;155(11):e202213315. doi: 10.1085/jgp.202213315. Epub 2023 Sep 19. J Gen Physiol. 2023. PMID: 37725091 Free PMC article.
-
Cardiac contractility: how calcium activates the myofilaments.Naturwissenschaften. 1998 Dec;85(12):575-82. doi: 10.1007/s001140050554. Naturwissenschaften. 1998. PMID: 9871917 Review.
-
Myosin crossbridge activation of cardiac thin filaments: implications for myocardial function in health and disease.Circ Res. 2004 May 28;94(10):1290-300. doi: 10.1161/01.RES.0000127125.61647.4F. Circ Res. 2004. PMID: 15166116 Review.
Cited by
-
Modeling cardiac contractile cooperativity across species.J Gen Physiol. 2025 Mar 3;157(2):e202413722. doi: 10.1085/jgp.202413722. Epub 2025 Jan 31. J Gen Physiol. 2025. PMID: 39887987
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

