Valproate discontinuation in girls and women of childbearing age with epilepsy: An Italian multicenter retrospective study on prescribing patterns and outcomes
- PMID: 39869104
- PMCID: PMC12097465
- DOI: 10.1111/epi.18281
Valproate discontinuation in girls and women of childbearing age with epilepsy: An Italian multicenter retrospective study on prescribing patterns and outcomes
Abstract
Objective: This study aimed to identify prescribing behaviors in women of childbearing potential (WOCP) with epilepsy already taking valproate (VPA), and to investigate the relationship between VPA maintenance, substitution, reduction, or withdrawal as part of polytherapy, and seizure worsening or relapse.
Methods: We retrospectively reviewed the prescription behaviors and seizure outcomes in WOCP (16-50 years of age) with epilepsy, referred to eight Italian epilepsy centers, who were taking VPA for at least 1 year between 2014 and 2019.
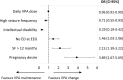
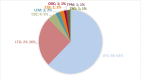
Results: Among 750 women (~12% of all WOCP), 528 (70.4%) maintained VPA unchanged throughout the observation period, 103 (13.7%) replaced VPA with another antiseizure medication (ASM), 90 (12%) reduced VPA, and 29 (3.9%) discontinued VPA in polytherapy. Focal epilepsy was most strongly associated with VPA withdrawal (odds ratio [OR] 2.96, 95% confidence interval [CI] 1.38-6.38), whereas generalized epilepsy was most associated with its non-withdrawal (reduction/switch/maintenance) (OR .31, 95% CI .14-.68). Intellectual disability, higher seizure frequency, and higher VPA doses were linked to VPA continuation. VPA withdrawal from polytherapy was associated with a higher risk of tonic-clonic seizure worsening (OR 2.91, 95% CI 1.09-7.77) compared to non-withdrawal.
Significance: VPA was rarely withdrawn or substituted in WOCP with epilepsy, in secondary and tertiary care settings following European regulatory restrictions. This likely reflects a population with severe epilepsies where VPA is difficult to replace; whereas women with milder epilepsies likely discontinued VPA earlier, as evidenced by its low overall prescription frequency. Withdrawal of VPA from a polytherapy regimen was associated with a threefold increased risk of seizure exacerbation.
Keywords: focal epilepsy; generalized epilepsy; tonic‐clonic seizures.
© 2025 The Author(s). Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
Roberta Esposto received support for attending meetings from Angelini Pharma. Giovanni Falcicchio received support for attending meetings from Angelini Pharma and UCB. Elena Zambrelli received travel support from Eisai, Angelini Pharma, and Lusofarmaco; and speaker honoraria from Italfarmaco, Eisai, and Jazz Pharma. Emanuele CerulliIrelli reports speaking honoraria from Lusofarmaco and travel support from UCB Pharma and Angelini Pharma. Federica Ranzato received speaker fees from Eisai, UCB, and LivaNova. Loretta Giuliano reports speaking honoraria from Lusofarmaco and travel support from Angelini Pharma, Ecupharma, and Eisai. Carlo Aandrea Galimberti received personal compensation for serving on a scientific advisory board from BIAL‐Portela & CaS.A (2014); for data safety monitoring board from UCB Pharma (2016); honoraria for speaking engagements from UCB Pharma (2016–2017), Sanofi (2018), Sandoz s.p.a. (2018), Eisai (2019–2022), Lusofarmaco (2020–2024), and Angelini Pharma (2021–2023); and research support paid to Mondino Foundation from UCB Pharma (as Investigator and Expert—2014; as Principal Investigator—2020), BIAL‐Portela & CaS.A (as Principal Investigator—2014), and the Italian Ministry of Health (RF 2008). Angela La Neve received speaker or consultancy fees from Eisai, Mylan, Sanofi, Bial, GW, UCB Pharma, Arvelle Therapeutics, Angelini Pharma, and Neuraxpharm. Diana Polo. received speaker fees from Eisai. Francesca Bisulli received consulting fees from Angelini Pharma, Eisai, Takeda, and UCB, and travel support from LivaNova. Barbara Mostacci reports speaking honoraria or personal fees from Eisai and LivaNova, and congress or travel support from Eisai and GW Jazz Pharma. The remaining authors have no conflicts of interest.
Figures
References
-
- Glauser T, Ben‐Menachem E, Bourgeois B, Cnaan A, Guerreiro C, Kälviäinen R, et al. Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2013;54(3):551–563. - PubMed
-
- Perucca E, Tomson T. The pharmacological treatment of epilepsy in adults. The Lancet Neurol. 2011;10:446–456. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

