Variable treatment response to lumasiran in pediatric patients with primary hyperoxaluria type 1
- PMID: 39869204
- PMCID: PMC12031841
- DOI: 10.1007/s00467-025-06665-w
Variable treatment response to lumasiran in pediatric patients with primary hyperoxaluria type 1
Abstract
Background: Primary hyperoxaluria type 1 (PH 1) is a rare genetic condition due to mutations in the AGXT gene. This leads to an overproduction of oxalate in the liver. Hyperoxaluria often causes kidney stones, nephrocalcinosis, and chronic kidney disease. Lumasiran is a recently approved drug that reduces the hepatic oxalate production by mRNA interference.
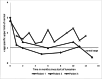
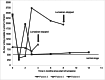
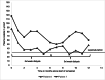
Methods: In this multicenter study, we evaluated the response to lumasiran treatment in PH 1 patients (n = 8) with a median age of 10.9 years (range 1.2-17.9 years), including two patients on hemodialysis. We retrospectively analyzed the reduction of urinary and plasma oxalate levels as well as changes in kidney stone events, nephrocalcinosis, and kidney function.
Results: In patients without kidney failure, the median reduction of urinary oxalate was 64% (range 10-80%) and 71% (61-86%) at 6 and 12 months, respectively. However, only one patient reached urinary oxalate levels within the age-specific normal range. Two patients did not respond to lumasiran and treatment was stopped. In one of the two patients on hemodialysis, the frequency of sessions could be reduced. The only notable side effects were injection site reactions.
Conclusion: There was a variable response to lumasiran in PH 1. Despite a reduction of hyperoxaluria in many patients with PH 1, only one patient reached normal values and 2 of 8 patients did not respond. Regular monitoring of urinary oxalate values and registry data collection seems mandatory to monitor the efficacy and the long-term outcome of PH 1 treated with lumasiran.
Keywords: Kidney function; Kidney stones; Nephrocalcinosis; Oxalate; RNA interference.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: MJK received consulting fees for an expert meeting from Alnylam. NK participates regularly in advisory boards for Alnylam. All other authors have no competing interests to declare that are relevant to the content of this article.
Figures

References
-
- Cochat P, Rumsby G (2013) Primary hyperoxaluria. N Engl J Med 369:649–658. 10.1056/NEJMra1301564 - PubMed
-
- Danpure CJ, Jennings PR, Fryer P, Purdue PE, Allsop J (1994) Primary hyperoxaluria type 1: genotypic and phenotypic heterogeneity. J Inherit Metab Dis 17:487–499. 10.1007/bf00711363 - PubMed
-
- Bacchetta J, Boivin G, Cochat P (2016) Bone impairment in primary hyperoxaluria: a review. Pediatr Nephrol 31:1–6. 10.1007/s00467-015-3048-z - PubMed
-
- Birtel J, Herrmann P, Garrelfs SF, Dulz S, Atiskova Y, Diederen RM et al (2019) The ocular phenotype in primary hyperoxaluria type 1. Am J Ophthalmol 206:184–191. 10.1016/j.ajo.2019.04.036 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

