Daytime DNase-I Administration Protects Mice From Ischemic Stroke Without Inducing Bleeding or tPA-Induced Hemorrhagic Transformation, Even With Aspirin Pretreatment
- PMID: 39869712
- PMCID: PMC11771350
- DOI: 10.1161/STROKEAHA.124.049961
Daytime DNase-I Administration Protects Mice From Ischemic Stroke Without Inducing Bleeding or tPA-Induced Hemorrhagic Transformation, Even With Aspirin Pretreatment
Abstract
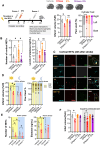
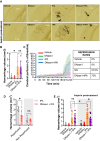
Background: Acute ischemic stroke treatment typically involves tissue-type plasminogen activator (tPA) or tenecteplase, but about 50% of patients do not achieve successful reperfusion. The causes of tPA resistance, influenced by thrombus composition and timing, are not fully clear. Neutrophil extracellular traps (NETs), associated with poor outcomes and reperfusion resistance, contribute to thrombosis. DNase-I, which degrades neutrophil extracellular traps, could improve thrombolytic efficacy. However, more studies are needed to understand the impact of DNase-I in tPA-sensitive stroke models, the safety of coadministering DNase-I and tPA regarding hemorrhagic transformation (HT), optimal timing for use, and effects on aspirin-treated animals.
Methods: We used in situ thromboembolic stroke, a tPA-sensitive model, where late tPA administration causes HT. Middle cerebral artery occlusion was induced at different zeitgeber times (ZT) to study the optimal timing for administration. DNase-I, tPA, and aspirin were administered at various times to evaluate their effects.
Results: DNase-I reduced infarct volume and improved functional outcomes 24 hours post-middle cerebral artery occlusion by decreasing plasma and cortical neutrophil extracellular trap levels. DNase-I caused no bleeding or impact on HT induced by late tPA. Its protective effect was only seen when given during the daytime (rodent inactive phase; ZT4-7), not overnight (active phase; ZT13-16). Chronic aspirin pretreatment increased tPA-induced HT but did not change the protective effects of DNase-I, with or without tPA.
Conclusions: Our study demonstrates that daytime (inactive phase) DNase-I administration is a safe and effective treatment for experimental stroke. This is particularly important given the 2 ongoing clinical trials for stroke patients.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT05203224 and NCT05880524.
Keywords: aspirin; extracellular traps; ischemic stroke; thrombosis; tissue-type plasminogen activator.
Conflict of interest statement
None.
Figures
Similar articles
-
Asparagine Endopeptidase Inhibition Attenuates Tissue Plasminogen Activator-Induced Brain Hemorrhagic Transformation After Ischemic Stroke.CNS Neurosci Ther. 2025 Mar;31(3):e70345. doi: 10.1111/cns.70345. CNS Neurosci Ther. 2025. PMID: 40116141 Free PMC article.
-
Thrombus Neutrophil Extracellular Traps Content Impair tPA-Induced Thrombolysis in Acute Ischemic Stroke.Stroke. 2018 Mar;49(3):754-757. doi: 10.1161/STROKEAHA.117.019896. Epub 2018 Feb 8. Stroke. 2018. PMID: 29438080 Clinical Trial.
-
Neutrophil extracellular traps promote tPA-induced brain hemorrhage via cGAS in mice with stroke.Blood. 2021 Jul 8;138(1):91-103. doi: 10.1182/blood.2020008913. Blood. 2021. PMID: 33881503 Free PMC article.
-
Antithrombotic Agents for tPA-Induced Cerebral Hemorrhage: A Systematic Review and Meta-Analysis of Preclinical Studies.J Am Heart Assoc. 2020 Dec 15;9(24):e017876. doi: 10.1161/JAHA.120.017876. Epub 2020 Dec 5. J Am Heart Assoc. 2020. PMID: 33283576 Free PMC article.
-
Efficacy of Chinese herbal medicine for tPA thrombolysis in experimental stroke: A systematic review and meta-analysis.Phytomedicine. 2022 Jun;100:154072. doi: 10.1016/j.phymed.2022.154072. Epub 2022 Mar 23. Phytomedicine. 2022. PMID: 35349833
Cited by
-
NETosis in myocardial ischemia-reperfusion injury: From mechanisms to therapies (Review).Biomed Rep. 2025 May 13;23(1):113. doi: 10.3892/br.2025.1991. eCollection 2025 Jul. Biomed Rep. 2025. PMID: 40420974 Free PMC article. Review.
References
-
- Jang IK, Gold HK, Ziskind AA, Fallon JT, Holt RE, Leinbach RC, May JW, Collen D. Differential sensitivity of erythrocyte-rich and platelet-rich arterial thrombi to lysis with recombinant tissue-type plasminogen activator. A possible explanation for resistance to coronary thrombolysis. Circulation. 1989;79:920–928. doi: 10.1161/01.cir.79.4.920 - PubMed
-
- Vandelanotte S, François O, Desender L, Staessens S, Vanhoorne A, Van Gool F, Tersteeg C, Vanhoorelbeke K, Vanacker P, Andersson T, et al. . R-tPA resistance is specific for platelet-rich stroke thrombi and can be overcome by targeting nonfibrin components. Stroke. 2024;55:1181–1190. doi: 10.1161/STROKEAHA.123.045880 - PubMed
-
- Laridan E, Denorme F, Desender L, François O, Andersson T, Deckmyn H, Vanhoorelbeke K, De Meyer SF. Neutrophil extracellular traps in ischemic stroke thrombi. Ann Neurol. 2017;82:223–232. doi: 10.1002/ana.24993 - PubMed
-
- Peña-Martínez C, Durán-Laforet V, García-Culebras A, Ostos F, Hernández-Jiménez M, Bravo-Ferrer I, Pérez-Ruiz A, Ballenilla F, Díaz-Guzmán J, Pradillo JM, et al. . Pharmacological modulation of neutrophil extracellular traps reverses thrombotic stroke tPA (Tissue-Type Plasminogen Activator) resistance. Stroke. 2019;50:3228–3237. doi: 10.1161/STROKEAHA.119.026848 - PubMed
-
- García-Yébenes I, Sobrado M, Zarruk JG, Castellanos M, Pérez de la Ossa N, Dávalos A, Serena J, Lizasoain I, Moro MA. A mouse model of hemorrhagic transformation by delayed tissue plasminogen activator administration after in situ thromboembolic stroke. Stroke. 2011;42:196–203. doi: 10.1161/STROKEAHA.110.600452 - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

