Prevalence of Pathogenic Variants and Eligibility Criteria for Genetic Testing in Patients Who Visit a Memory Clinic
- PMID: 39869842
- PMCID: PMC11776143
- DOI: 10.1212/WNL.0000000000210273
Prevalence of Pathogenic Variants and Eligibility Criteria for Genetic Testing in Patients Who Visit a Memory Clinic
Abstract
Background and objectives: Identifying genetic causes of dementia in patients visiting memory clinics is important for patient care and family planning. Traditional clinical selection criteria for genetic testing may miss carriers of pathogenic variants in dementia-related genes. This study aimed identify how many carriers we are missing and to optimize criteria for selecting patients for genetic counseling in memory clinics.
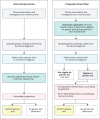
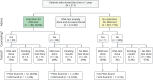
Methods: In this clinical cohort study, we retrospectively genetically tested patients during 2.5 years (2010-2012) visiting the Alzheimer Center Amsterdam, a specialized memory clinic. Genetic tests consisted of a 54-gene dementia panel, focusing on Class IV/V variants per American College of Medical Genetics and Genomics guidelines, including APP duplications and the C9ORF72 repeat expansion. We determined the prevalence of pathogenic variants and propose new eligibility criteria for genetic testing in memory clinics. The eligibility criteria were prospectively applied for 1 year (2021-2022), and results were compared with the retrospective cohort.
Results: Genetic tests were retrospectively performed in in 1,022 of 1,138 patients (90%) who consecutively visited the memory clinic. Among these, 1,022 patients analyzed (mean age 62.1 ± 8.9 years; 40.4% were female), 34 pathogenic variant carriers were identified (3.3%), with 24 being symptomatic. Previous clinical criteria would have identified only 15 carriers (44% of all carriers, 65% of symptomatic carriers). The proposed criteria increased identification to 22 carriers (62.5% of all carriers, 91% of symptomatic carriers). In the prospective cohort, 148 (28.7%) of 515 patients were eligible for testing under the new criteria. Of the 90 eligible patients who consented to testing, 13 pathogenic carriers were identified, representing a 73% increase compared with the previous criteria.
Discussion: We found that patients who visit a memory clinic and carry a pathogenic genetic variant are often not eligible for genetic testing. The proposed new criteria improve the identification of patients with a genetic cause for their cognitive complaints. In systems without practical or financial barriers to genetic testing, the new criteria can enhance personalized care. In other countries where the health care systems differs and in other genetic ancestry groups, the performance of the criteria may be different.
Conflict of interest statement
S.J. Van Der Lee, M. Hulsman, and R. Van Spaendonk report no disclosures relevant to the manuscript. J. Van Der Schaar wrote a book for a layman's audience about the personal impact of dominantly inherited AD, for which she received grants or contracts from Aegon Nederland and Alzheimer Nederland and royalties from Uitgeverij Prometheus. She is a member of the advisory board for the National Dementia Strategy of the Dutch Ministry of Health, Welfare and Sport. J. Dijkstra, N. Tesi, F. van Ruissen, M. Elting, M. Reinders, I. De Rojas, and C.C. Verschuuren-Bemelmans report no disclosures relevant to the manuscript. W.M. Van Der Flier has research programs that have been funded by ZonMW, NWO, EU-JPND, EU-IHI, Alzheimer Nederland, Hersenstichting CardioVascular Onderzoek Nederland, Health∼Holland, Topsector Life Sciences & Health, Stichting Dioraphte, Gieskes-Strijbis Fonds, Stichting Equilibrio, Edwin Bouw Fonds, Pasman Stichting, Stichting Alzheimer & Neuropsychiatrie Foundation, Philips, Biogen MA Inc., Novartis-NL, Life-MI, AVID, Roche BV, Fujifilm, Eisai, and Combinostics; she has been an invited speaker at Biogen MA Inc., Danone, Eisai, WebMD Neurology (Medscape), NovoNordisk, Springer Healthcare, European Brain Council; all funding is paid to her institution; she has been an invited speaker at Biogen MA Inc., Danone, Eisai, WebMD Neurology (Medscape), NovoNordisk, Springer Healthcare, European Brain Council; all funding is paid to her institution; she is consultant to Oxford Health Policy Forum CIC, Roche, Biogen MA Inc., and Eisai; all funding is paid to her institution; she participated in advisory boards of Biogen MA Inc., Roche, and Eli Lilly; she is a member of the steering committee of EVOKE/EVOKE+ (NovoNordisk); all funding is paid to her institution; she is member of the steering committee of PAVE, and Think Brain Health; she was associate editor of
Figures


References
-
- Aalfs CM, Vervenne-van Spaendonk R, Pijnenburg YAL, Cohn-Hokke PE, Meijers HJ, Scheltens P. DNA diagnostics in dementia [in Dutch]. Ned Tijdschr Geneeskd. 2017;161:D1774. - PubMed
-
- Richards S, Aziz N, Bale S, et al. . Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. doi:10.1038/gim.2015.30 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
