A role for mitochondria-ER crosstalk in amyotrophic lateral sclerosis 8 pathogenesis
- PMID: 39870504
- PMCID: PMC11772500
- DOI: 10.26508/lsa.202402907
A role for mitochondria-ER crosstalk in amyotrophic lateral sclerosis 8 pathogenesis
Abstract
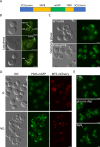
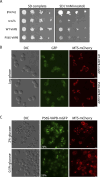
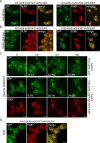
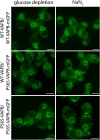
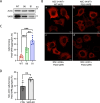
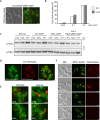
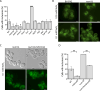
Protein aggregates in motoneurons, a pathological hallmark of amyotrophic lateral sclerosis, have been suggested to play a key pathogenetic role. ALS8, characterized by ER-associated inclusions, is caused by a heterozygous mutation in VAPB, which acts at multiple membrane contact sites between the ER and almost all other organelles. The link between protein aggregation and cellular dysfunction is unclear. A yeast model, expressing human mutant and WT-VAPB under the control of the orthologous yeast promoter in haploid and diploid cells, was developed to mimic the disease situation. Inclusion formation was found to be a developmentally regulated process linked to mitochondrial damage that could be attenuated by reducing ER-mitochondrial contacts. The co-expression of the WT protein retarded P56S-VAPB inclusion formation. Importantly, we validated these results in mammalian motoneuron cells. Our findings indicate that (age-related) damage to mitochondria influences the propensity of the mutant VAPB to form aggregates via ER-mitochondrial contacts, initiating a series of events leading to disease progression.
© 2025 Wilson et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





Similar articles
-
Yeast Models of Amyotrophic Lateral Sclerosis Type 8 Mimic Phenotypes Seen in Mammalian Cells Expressing Mutant VAPBP56S.Biomolecules. 2023 Jul 19;13(7):1147. doi: 10.3390/biom13071147. Biomolecules. 2023. PMID: 37509182 Free PMC article.
-
A VAPB mutant linked to amyotrophic lateral sclerosis generates a novel form of organized smooth endoplasmic reticulum.FASEB J. 2010 May;24(5):1419-30. doi: 10.1096/fj.09-147850. Epub 2009 Dec 14. FASEB J. 2010. PMID: 20008544
-
Vapb/Amyotrophic lateral sclerosis 8 knock-in mice display slowly progressive motor behavior defects accompanying ER stress and autophagic response.Hum Mol Genet. 2015 Nov 15;24(22):6515-29. doi: 10.1093/hmg/ddv360. Epub 2015 Sep 11. Hum Mol Genet. 2015. PMID: 26362257 Free PMC article.
-
The Link between VAPB Loss of Function and Amyotrophic Lateral Sclerosis.Cells. 2021 Jul 23;10(8):1865. doi: 10.3390/cells10081865. Cells. 2021. PMID: 34440634 Free PMC article. Review.
-
ER stress and unfolded protein response in amyotrophic lateral sclerosis.Mol Neurobiol. 2009 Apr;39(2):81-9. doi: 10.1007/s12035-009-8054-3. Epub 2009 Jan 30. Mol Neurobiol. 2009. PMID: 19184563 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
