METTL1 coordinates cutaneous squamous cell carcinoma progression via the m7G modification of the ATF4 mRNA
- PMID: 39870616
- PMCID: PMC11772585
- DOI: 10.1038/s41420-025-02304-3
METTL1 coordinates cutaneous squamous cell carcinoma progression via the m7G modification of the ATF4 mRNA
Abstract
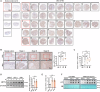
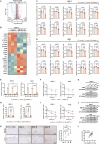
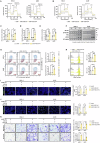
Methyltransferase-like 1 (METTL1)-mediated m7G modification is a common occurrence in various RNA species, including mRNAs, tRNAs, rRNAs, and miRNAs. Recent evidence suggests that this modification is linked to the development of several cancers, making it a promising target for cancer therapy. However, the specific role of m7G modification in cutaneous squamous cell carcinoma (cSCC) is not well understood. In this study, we observed conspicuously elevated levels of METTL1 in cSCC tumors and cell lines. Inhibiting METTL1 led to reduced survival, migration, invasion, and xenograft tumor growth in cSCC cells. Mechanistically, through a combination of RNA sequencing, m7G methylated immunoprecipitation (MeRIP)-qPCR, and mRNA stability assays, we discovered that METTL1 is responsible for the m7G modification of ATF4 mRNA, leading to increased expression of ATF4. Importantly, we demonstrated that this modification is dependent on the methyltransferase activity of METTL1. Additionally, we observed a positive association between ATF4 expression and METTL1 levels in cSCC tumors. Intriguingly, restoring ATF4 expression in cSCC cells not only promoted glycolysis but also reversed the anti-tumor effects of METTL1 knockdown. In conclusion, our results underscore the critical role of METTL1 and m7G modification in cSCC tumorigenesis, suggesting a promising target for future cSCC therapies.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethical approval and consent to participate: All the experiments related to human tissues were conducted in accordance with Helsinki criteria and were approved by the Ethics Committees of Shanghai Outdo Biotech Co. Ltd (approval number SHYJS-CP-2001009). All mice studies are conducted according to protocols approved by the Animal Ethics Committee of Anhui Medical University with the approval number LLSC20240910.
Figures




References
-
- Winge MCG, Kellman LN, Guo K, Tang JY, Swetter SM, Aasi SZ, et al. Advances in cutaneous squamous cell carcinoma. Nat Rev Cancer. 2023;23:430–49. - PubMed
-
- Knackstedt TJ, Knackstedt RW, Djohan M, Djohan R, Gastman BR, Crowe DR. New Developments in the Management of Cutaneous Squamous Cell Carcinoma. Plast Reconstr Surg. 2021;147:492–504. - PubMed
LinkOut - more resources
Full Text Sources

