Stromal architecture and fibroblast subpopulations with opposing effects on outcomes in hepatocellular carcinoma
- PMID: 39870619
- PMCID: PMC11772884
- DOI: 10.1038/s41421-024-00747-z
Stromal architecture and fibroblast subpopulations with opposing effects on outcomes in hepatocellular carcinoma
Abstract
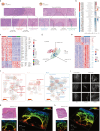
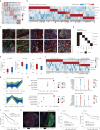
Dissecting the spatial heterogeneity of cancer-associated fibroblasts (CAFs) is vital for understanding tumor biology and therapeutic design. By combining pathological image analysis with spatial proteomics, we revealed two stromal archetypes in hepatocellular carcinoma (HCC) with different biological functions and extracellular matrix compositions. Using paired single-cell RNA and epigenomic sequencing with Stereo-seq, we revealed two fibroblast subsets CAF-FAP and CAF-C7, whose spatial enrichment strongly correlated with the two stromal archetypes and opposing patient prognosis. We discovered two functional units, one is the intratumor inflammatory hub featured by CAF-FAP plus CD8_PDCD1 proximity and the other is the marginal wound-healing hub with CAF-C7 plus Macrophage_SPP1 co-localization. Inhibiting CAF-FAP combined with anti-PD-1 in orthotopic HCC models led to improved tumor regression than either monotherapy. Collectively, our findings suggest stroma-targeted strategies for HCC based on defined stromal archetypes, raising the concept that CAFs change their transcriptional program and intercellular crosstalk according to the spatial context.
© 2025. The Author(s).
Conflict of interest statement
Conflict of interest: The authors declare no competing interests.
Figures





References
-
- Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71, 209–249 (2021). - PubMed
-
- Llovet, J. M. et al. Hepatocellular carcinoma. Nat. Rev. Dis. Prim.7, 6 (2021). - PubMed
-
- Haber, P. K. et al. Molecular markers of response to anti-PD1 therapy in advanced hepatocellular carcinoma. Gastroenterology164, 72–88.e18 (2023). - PubMed
-
- Kisseleva, T. & Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol.18, 151–166 (2021). - PubMed
Grants and funding
- U23A6010/National Natural Science Foundation of China (National Science Foundation of China)
- 82394450/National Natural Science Foundation of China (National Science Foundation of China)
- 82103037/National Natural Science Foundation of China (National Science Foundation of China)
- 82090053/National Natural Science Foundation of China (National Science Foundation of China)
- 82203643/National Natural Science Foundation of China (National Science Foundation of China)
- 82172860/National Natural Science Foundation of China (National Science Foundation of China)
- 82130077/National Natural Science Foundation of China (National Science Foundation of China)
- 81961128025/National Natural Science Foundation of China (National Science Foundation of China)
- 32370978/National Natural Science Foundation of China (National Science Foundation of China)
- 22YF1407200/Science and Technology Commission of Shanghai Municipality (Shanghai Municipal Science and Technology Commission)
- 19XD1420700/Science and Technology Commission of Shanghai Municipality (Shanghai Municipal Science and Technology Commission)
- 20XD1424900/Science and Technology Commission of Shanghai Municipality (Shanghai Municipal Science and Technology Commission)
- 2018SHZDZX05/Science and Technology Commission of Shanghai Municipality (Shanghai Municipal Science and Technology Commission)
- 2020YFA0509000/Ministry of Science and Technology of the People's Republic of China (Chinese Ministry of Science and Technology)
- 2022YFA1302902/Ministry of Science and Technology of the People's Republic of China (Chinese Ministry of Science and Technology)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

