Phosphorylation-dependent WRN-RPA interaction promotes recovery of stalled forks at secondary DNA structure
- PMID: 39870632
- PMCID: PMC11772831
- DOI: 10.1038/s41467-025-55958-z
Phosphorylation-dependent WRN-RPA interaction promotes recovery of stalled forks at secondary DNA structure
Abstract
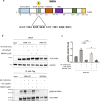
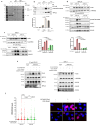
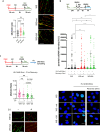
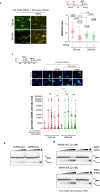
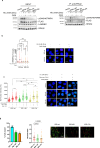
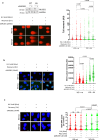
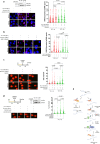
The WRN protein is vital for managing perturbed replication forks. Replication Protein A strongly enhances WRN helicase activity in specific in vitro assays. However, the in vivo significance of RPA binding to WRN has largely remained unexplored. We identify several conserved phosphorylation sites in the acidic domain of WRN targeted by Casein Kinase 2. These phosphorylation sites are crucial for WRN-RPA interaction. Using an unphosphorylable WRN mutant, which lacks the ability to bind RPA, we determine that the WRN-RPA complex plays a critical role in fork recovery after replication stress countering the persistence of G4 structures after fork stalling. However, the interaction between WRN and RPA is not necessary for the processing of replication forks when they collapse. The absence of WRN-RPA binding hampers fork recovery, causing single-strand DNA gaps, enlarged by MRE11, and triggering MUS81-dependent double-strand breaks, which require repair by RAD51 to prevent excessive DNA damage.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


Update of
-
PHOSPHORYLATION-DEPENDENT ASSOCIATION OF WRN WITH RPA IS REQUIRED FOR RECOVERY OF REPLICATION FORKS STALLED AT SECONDARY DNA STRUCTURES.bioRxiv [Preprint]. 2023 Aug 9:2023.08.08.552428. doi: 10.1101/2023.08.08.552428. bioRxiv. 2023. Update in: Nat Commun. 2025 Jan 27;16(1):997. doi: 10.1038/s41467-025-55958-z. PMID: 37609214 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

