Time in therapeutic range of tacrolimus in allogeneic hematopoietic stem cell transplant recipients is associated with acute graft-versus-host disease prophylaxis
- PMID: 39870810
- PMCID: PMC11772651
- DOI: 10.1038/s41598-025-87801-2
Time in therapeutic range of tacrolimus in allogeneic hematopoietic stem cell transplant recipients is associated with acute graft-versus-host disease prophylaxis
Abstract
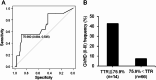
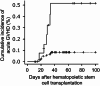
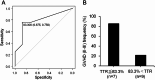
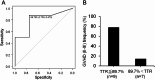
Intra-patient variability in immunosuppressive blood drug concentrations is a potential biomarker in managing organ transplant patients. However, the association between the time in therapeutic range of tacrolimus blood concentrations and its efficacy in preventing graft-versus-host disease remains unknown. In this study, we analyzed the relationship between the time in therapeutic range of tacrolimus blood concentrations and its efficacy in acute graft-versus-host disease prophylaxis in patients undergoing allogeneic hematopoietic stem cell transplantation. Eligible patients administered tacrolimus were categorized into two groups based on the grade of acute graft-versus-host disease, and propensity score matching was performed using graft-versus-host disease prophylaxis protocols and days to the disease onset to compare time in therapeutic range. In patients with tacrolimus blood concentration therapeutic range ≥ 10 ng/mL, time in therapeutic range during the first 4 weeks post-transplantation was significantly lower in the Grade II-III than in the Grade 0-I group. Among propensity score matching-extracted patients, the Grade II-III group had significantly lower time in therapeutic range during the first 2 and 4 weeks post-transplantation. Our results suggest that high time in therapeutic range early post-transplantation, particularly within 4 weeks, may avert the severity of acute graft-versus-host disease.
Keywords: Graft-versus-host disease; Hematopoietic stem cell transplantation; Tacrolimus; Therapeutic drug monitoring; Time in therapeutic range.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
References
-
- Thomson, A. W., Bonham, C. A. & Zeevi, A. Mode of action of tacrolimus (FK506): molecular and cellular mechanisms. Ther. Drug Monit.17, 584–591 (1995). - PubMed
-
- Plosker, G. L. & Foster, R. H. Tacrolimus: A further update of its pharmacology and therapeutic use in the management of organ transplantation. Drugs59, 323–389 (2000). - PubMed
-
- Przepiorka, D. et al. Relationship of tacrolimus whole blood levels to efficacy and safety outcomes after unrelated donor marrow transplantation. Biol. Blood Marrow Transpl.5, 94–97 (1999). - PubMed
-
- Gao, Y. & Ma, J. Tacrolimus in adult hematopoietic stem cell transplantation. Expert Opin. Drug Metab. Toxicol.15, 803–811 (2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

