Engineered Passive Glucose Uptake in Pseudomonas taiwanensis VLB120 Increases Resource Efficiency for Bioproduction
- PMID: 39871105
- PMCID: PMC11772102
- DOI: 10.1111/1751-7915.70095
Engineered Passive Glucose Uptake in Pseudomonas taiwanensis VLB120 Increases Resource Efficiency for Bioproduction
Abstract
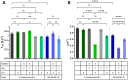
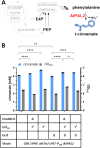
Glucose is the most abundant monosaccharide and a principal substrate in biotechnological production processes. In Pseudomonas, this sugar is either imported directly into the cytosol or first oxidised to gluconate in the periplasm. While gluconate is taken up via a proton-driven symporter, the import of glucose is mediated by an ABC-type transporter, and hence both require energy. In this study, we heterologously expressed the energy-independent glucose facilitator protein (Glf) from Zymomonas mobilis to replace the native energy-demanding glucose transport systems, thereby increasing the metabolic energy efficiency. The implementation of passive glucose uptake in engineered production strains significantly increased product titres and yields of the two different aromatic products, cinnamic acid (+10%-15%) and resveratrol (+26%; 18.1 mg/g) in batch cultures.
Keywords: Pseudomonas; ATP consumption; glucose transport; metabolic engineering; strain optimization.
© 2025 The Author(s). Microbial Biotechnology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Expression of a xylose-specific transporter improves ethanol production by metabolically engineered Zymomonas mobilis.Appl Microbiol Biotechnol. 2014 Aug;98(15):6897-905. doi: 10.1007/s00253-014-5812-6. Epub 2014 May 17. Appl Microbiol Biotechnol. 2014. PMID: 24839214
-
An evolved xylose transporter from Zymomonas mobilis enhances sugar transport in Escherichia coli.Microb Cell Fact. 2009 Dec 15;8:66. doi: 10.1186/1475-2859-8-66. Microb Cell Fact. 2009. PMID: 20003468 Free PMC article.
-
Characterization of the Zymomonas mobilis glucose facilitator gene product (glf) in recombinant Escherichia coli: examination of transport mechanism, kinetics and the role of glucokinase in glucose transport.Mol Microbiol. 1995 Mar;15(5):795-802. doi: 10.1111/j.1365-2958.1995.tb02350.x. Mol Microbiol. 1995. PMID: 7596282
-
New technologies provide more metabolic engineering strategies for bioethanol production in Zymomonas mobilis.Appl Microbiol Biotechnol. 2019 Mar;103(5):2087-2099. doi: 10.1007/s00253-019-09620-6. Epub 2019 Jan 19. Appl Microbiol Biotechnol. 2019. PMID: 30661108 Review.
-
Advances and prospects in metabolic engineering of Zymomonas mobilis.Metab Eng. 2018 Nov;50:57-73. doi: 10.1016/j.ymben.2018.04.001. Epub 2018 Apr 5. Metab Eng. 2018. PMID: 29627506 Review.
References
-
- Bujdoš, D. , Popelářová B., Volke D. C., Nikel P. I., Sonnenschein N., and Dvořák P.. 2023. “Engineering of Pseudomonas putida for Accelerated Co‐Utilization of Glucose and Cellobiose Yields Aerobic Overproduction of Pyruvate Explained by an Upgraded Metabolic Model.” Metabolic Engineering 75: 29–46. 10.1016/j.ymben.2022.10.011. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

