DLAT is involved in ovarian cancer progression by modulating lipid metabolism through the JAK2/STAT5A/SREBP1 signaling pathway
- PMID: 39871246
- PMCID: PMC11773875
- DOI: 10.1186/s12935-025-03656-7
DLAT is involved in ovarian cancer progression by modulating lipid metabolism through the JAK2/STAT5A/SREBP1 signaling pathway
Abstract
Background: Ovarian cancer (OC) remains a lethal gynecological malignancy with an alarming mortality rate, primarily attributed to delayed diagnosis and a lack of effective treatment modalities. Accumulated evidence highlights the pivotal role of reprogrammed lipid metabolism in fueling OC progression, however, the intricate underlying molecular mechanisms are not fully elucidated.
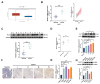
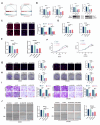
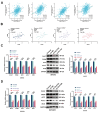
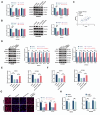
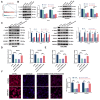
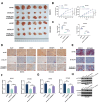
Methods: DLAT expression was assessed in OC tissues and cell lines by immunohistochemistry, western blot and qRT-PCR analysis. The effects of DLAT silencing on changes in lipid metabolism, cell viability, migration, and invasion were examined in SKOV3 and OVCAR3 cells using CCK-8, colony formation, Transwell migration and invasion, and wound-healing assays. GSEA analysis was used to examine the relationship between DLAT and lipid metabolism-related enzymes. Rescue experiments in which SREBP1 was overexpressed in DLAT-silenced cells were carried out. Western blot analysis was performed to determine whether the JAK2/STAT5 signaling pathway was involved in DLAT-regulated SREBP1 expression. Commercially available triglyceride and cholesterol detection kits, as well as Nile Red and Oil red O staining were used to measure lipid metabolism. A subcutaneous tumor model was established in BALB/c mice to confirm the role of the DLAT/SREBP1 axis in OC growth and metastasis in vivo.
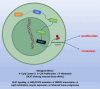
Results: DLAT expression was significantly upregulated in OC patient tissue and associated with poor prognosis. Silencing DLAT reduced lipid content and impaired OC cell proliferation, migration, and invasion. DLAT upregulated SREBP1 expression via the JAK2/STAT5 signaling pathway, enhancing expression of fatty acid synthesis enzymes and altering lipid metabolism. SREBP1 was essential for DLAT-dependent OC cell growth and metastasis both in vitro and in vivo.
Conclusion: This study uncovers a novel DLAT/JAK2/STAT5/SREBP1 axis that reprograms lipid metabolism in OC, providing insights into metabolic vulnerabilities and potential therapeutic targets for OC treatment.
Keywords: DLAT; JAK2/STAT5 signaling; Lipid metabolism; Ovarian cancer; SREBP1.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Ethics Committee of Obstetrics & Gynecology Hospital of Fudan University. All patients provided written informed consent prior to participation in the study. The study was conducted in accordance with the Declaration of Helsinki and followed the relevant institutional and national guidelines. All animal experiments were performed following protocols approved by the Institutional Animal Care and Use Committee of Obstetrics & Gynecology Hospital of Fudan University. Competing interests: The authors declare no competing interests.
Figures

References
-
- Webb PM, Jordan SJ. Global epidemiology of epithelial ovarian cancer. Nat Reviews Clin Oncol. 2024;21(5):389–400. - PubMed
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. Cancer J Clin. 2023;73(1):17–48. - PubMed
-
- Saman S, Srivastava N, Yasir M, Chauhan I. A Comprehensive Review on Current treatments and challenges involved in the treatment of Ovarian Cancer. Curr Cancer Drug Targets. 2024;24(2):142–66. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

