hUC-MSC preserves erectile function by restoring mitochondrial mass of penile smooth muscle cells in a rat model of cavernous nerve injury via SIRT1/PGC-1a/TFAM signaling
- PMID: 39871297
- PMCID: PMC11773750
- DOI: 10.1186/s40659-024-00578-y
hUC-MSC preserves erectile function by restoring mitochondrial mass of penile smooth muscle cells in a rat model of cavernous nerve injury via SIRT1/PGC-1a/TFAM signaling
Abstract
Background: Cavernous nerve injury-induced erectile dysfunction (CNI-ED) is a common complication following radical prostatectomy and severely affects patients' quality of life. The mitochondrial impairment in corpus cavernosum smooth muscle cells (CCSMCs) may be an important pathological mechanism of CNI-ED. Previous studies have shown that transplantation of human adipose derived stem cells (ADSC) can alleviate CNI-ED in a rat model. However, little is known about the effect of human umbilical cord mesenchymal stem cells (hUC-MSC) on CNI-ED. It remains unclear whether hUC-MSC can ameliorate mitochondrial damage in CCSMCs. In this study, we aimed to investigate the impacts of hUC-MSC on the mitochondrial mass and function of CCSMCs, as well as elucidate its underlying molecular mechanism.
Methods: The CNI-ED rat model was established by bilaterally crushing cavernous nerves. Subsequently, hUC-MSC were transplanted into the cavernosum and ADSC were injected as a positive control group. Erectile function evaluation and histological detection were performed 4 weeks after cell transplantation. In vitro, CCSMCs underwent hypoxia and were then co-cultured with ADSC or hUC-MSC using a transwell system. The mitochondrial mass and function, as well as signaling pathways, were investigated. To explore the role of the SIRT1/PGC-1α/TFAM pathway in regulating mitochondrial biogenesis of CCSMCs, we knocked down SIRT1 by siRNA.
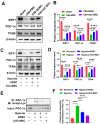
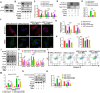
Results: The administration of hUC-MSC significantly improved erectile function of CNI-ED rats and reduced the ratio of collagen to smooth muscle. Specifically, hUC-MSC treatment restored mitochondrial mass and function in CCSMCs injured by CNI or hypoxia, and inhibited the apoptosis of CCSMCs. Mechanistically, the application of hUC-MSC activated SIRT1/PGC-1α/TFAM pathway both in rat penile tissues and CCSMCs. In addition, knockdown of SIRT1 in CCSMCs abolished the protective effects of hUC-MSC on mitochondrial mass and function, while leading to an increase in cellular apoptosis.
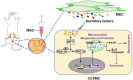
Conclusions: hUC-MSC contribute to the recovery of erectile function in CNI-ED rats by restoring mitochondrial mass and function of CCSMCs through the SIRT1/PGC-1α/TFAM pathway. Our present study offers new insights into the role and molecular mechanisms of hUC-MSC in regulating mitochondrial homeostasis, thereby facilitating the restoration of the erectile function in CNI-ED.
Keywords: Cavernous nerve injury-induced erectile dysfunction; Corpus cavernous smooth muscle cell; Mesenchymal stem cell; SIRT1/PGC-1α/TFAM pathway.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All experiments in this study conform to the Chinese legislation and have been approved by The Human and Animal Research Ethics Committee of Renji Hospital Affiliated to Shanghai Jiao Tong University School of Medicine. Consent for publication: Not applicable. Competing interests: The authors have declared that no conflict of interest exists.
Figures
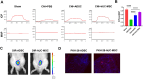
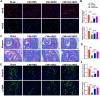
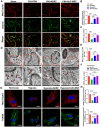
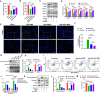
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

