FGF21 and APOA1 mRNA-based therapies for the treatment of experimental acute pancreatitis
- PMID: 39871339
- PMCID: PMC11773771
- DOI: 10.1186/s12967-025-06129-7
FGF21 and APOA1 mRNA-based therapies for the treatment of experimental acute pancreatitis
Abstract
Background: Acute pancreatitis (AP) presents a significant clinical challenge with limited therapeutic options. The complex etiology and pathophysiology of AP emphasize the need for innovative treatments. This study explores mRNA-based therapies delivering fibroblast growth factor 21 (FGF21) and apolipoprotein A1 (APOA1), alone and in combination, for treating experimental AP.
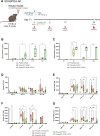
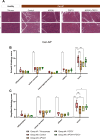
Methods: Liver-targeted lipid nanoparticles (LNP)-mRNA formulations encoding FGF21, APOA1, and a chimeric APOA1-FGF21, were first tested for protein expression and bioavailability in vitro and in mice fed a high-fat diet. Efficacy studies were performed in the caerulein-induced AP (Cer-AP) model, and a new AP model combining ethanol feeding with ethanol binge plus palmitoleic acid administration, the EtOH/POA-AP model. A single dose of the APOA1, FGF21, and APOA1-FGF21 LNP-mRNAs formulations was administered in both models. Serum levels of pancreatic lipase (LIPC), amylase (AMYL), and aspartate aminotransferase (AST), along with pancreatic tissue analyses using two histopathological scores were performed to evaluate treatment effects.
Results: In vitro studies demonstrated the translation and secretion of APOA1, FGF21, and APOA1-FGF21 proteins encoded by the LNP-mRNAs. In vivo, LNP-mRNA administration increased serum levels of the respective proteins in metabolically impaired (i.e. high fat diet-fed) mice. In the Cer-AP model, serum markers of pancreatic injury were similarly reduced when mice were treated with APOA1, FGF21, and APOA1-FGF21 LNP-mRNA, and this effect was also observed in the histopathological analyses. The EtOH/POA-AP model was more aggressive than the Cer-AP model. FGF21 and APOA1-FGF21 LNP-mRNAs were protective according to LIPC and AMYL serum levels, while APOA1 LNP-mRNA had little effect. On the other hand, histological improvements were more evident in mice receiving APOA1 LNP-mRNA. In the EtOH/POA-AP model, FGF21 and APOA1-FGF21 LNP-mRNAs reduced serum AST levels, indicating hepatoprotective activity.
Discussion: This proof-of-concept study demonstrates the potential of mRNA-based therapies delivering FGF21 and APOA1 in experimental AP. While individual treatments effectively reduced pancreatic injury, the APOA1-FGF21 fusion molecule did not exhibit superior activity. Liver-targeted LNP-mRNA administration may offer a promising approach for treating AP, leveraging endogenous production pathways for therapeutic proteins. Further research is warranted to elucidate the mechanisms underlying their therapeutic efficacy and optimize treatment regimens for clinical translation.
Keywords: APOA1; Acute pancreatitis; Cytoprotection; FGF21; Pancreatic injury; mRNA therapy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study received approval from the Ethics Committee for Animal Experimentation of the University of Navarra (protocol #065 − 22). Informed consent: Written informed consent was not applicable. Conflict of interest: Anne-Renee Graham holds stock in and is an employee of Moderna, Inc. The rest of the authors have no conflicts of interest to declare.
Figures





References
-
- Lankisch PG, Apte M, Banks PA, Acute pancreatitis. Lancet. 2015;386:85–96. 10.1016/S0140-6736(14)60649-8. - PubMed
-
- Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, et al. Acute pancreatitis. Lancet. 2020;396:726–34. 10.1016/S0140-6736(20)31310-6. - PubMed
-
- Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–11. 10.1136/GUTJNL-2012-302779. - PubMed
MeSH terms
Substances
Grants and funding
- Moderna Inc. US/Moderna Inc. US
- PI22/00147/Instituto de Salud Carlos III
- ARNMUNE Ref.: 0011-1411-2023-000032/Dirección General de Industria, Energia y Proyectos Estrategicos S3, Gobierno de Navarra
- RYC2018-024475-I funded by MCIU/AEI/10.13039/501100011033/Ministerio de Ciencia e Innovación
- Sara Borrell Contract CD22/00109/Ministerio de Sanidad, Consumo y Bienestar Social
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

