Improved primary stability and load transfer of a customized osseointegrated transfemoral prosthesis compared to a commercial one
- PMID: 39871350
- PMCID: PMC11770929
- DOI: 10.1186/s13018-025-05476-x
Improved primary stability and load transfer of a customized osseointegrated transfemoral prosthesis compared to a commercial one
Abstract
Background: Transfemoral osseointegrated prostheses, like other uncemented prostheses experience the risk of aseptic loosening and post-operative periprosthetic fractures, with an incidence between 3% and 30%. To date, however, osseointegrated off-the-shelf prostheses are manufactured in a limited number of sizes, and some patients do not meet the strict eligibility criteria of commercial devices. A customized osseointegrated stem was developed and a pre-clinical in vitro investigation of the stem was performed, to evaluate its biomechanical performance.
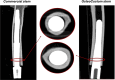
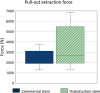
Materials and methods: Six human cadaveric femurs were implanted with commercial stems, while the six contralateral were implanted with customized stems. Three more femurs that did not meet the eligibility criteria for the commercial stems were implanted with the customized stems. Two different loading scenarios (compression-flexion, and torsion) were simulated to measure the primary implant stability and the load transfer. For both loading scenarios, the displacements of the implant with respect to the host bone, and the strains on the bone surface were measured using digital image correlation (DIC). To measure the pull-out force, a tensile force was applied to the prostheses.
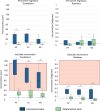
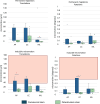
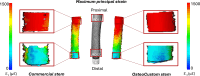
Results: The translational inducible micromotions during the compression-flexion test of the OsteoCustom stem were more than 4 times smaller than the commercial one (p < 0.05). The rotational inducible micromotions of the OsteoCustom stem were more than 3 times smaller than the commercial one (p < 0.05). Similar results were found from the torsional test. The full-field strain distribution of the commercial stem showed a slightly higher strain concentration near the stem tip (maximum principal strain = 1928±127 µɛ) than the OsteoCustom (maximum principal strain = 1758±130 µɛ). Similar results were found for the femurs that did not meet the eligibility criteria for the commercial stems and could be implanted with the OsteoCustom. No statistically significant difference was found in the extraction force between the two groups.
Discussion and conclusion: These results support the hypothesis that the OsteoCustom stem can offer better primary stability and load distribution compared to commercial implants. The outcome highlighted the potential benefits of the OsteoCustom prosthesis, which is capable of including a wider range of femoral anatomies than the current standard.
Keywords: In vitro biomechanical test; Implant micromotions; Lower-limb amputation; Primary stability; Stem-bone load transfer; Transfemoral osseointegrated prosthesis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The study complied with the Declaration of Helsinki and was approved by the Ethical Committee of the University of Bologna (reference n. 113063, 10th May 2021). The human cadaveric femurs were obtained through an ethically approved international donation program (Anatomy Gift Registry, USA). Competing interests: The authors declare no competing interests.
Figures



References
-
- Hughes W, Goodall R, Salciccioli JD, Marshall DC, Davies AH, Shalhoub J. Editor’s Choice – Trends in Lower Extremity Amputation Incidence in European Union 15 + Countries 1990–2017, Eur. J. Vasc. Endovasc. Surg., vol. 60, no. 4, pp. 602–612, Oct. 2020, 10.1016/j.ejvs.2020.05.037 - PubMed
-
- Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the Prevalence of Limb Loss in the United States: 2005 to 2050, Arch. Phys. Med. Rehabil., vol. 89, no. 3, pp. 422–429, Mar. 2008, 10.1016/j.apmr.2007.11.005 - PubMed
-
- Örgel M et al. Dec., Comparison of functional outcome and patient satisfaction between patients with socket prosthesis and patients treated with transcutaneous osseointegrated prosthetic systems (TOPS) after transfemoral amputation, Eur. J. Trauma Emerg. Surg., vol. 48, no. 6, pp. 4867–4876, 2022, 10.1007/s00068-022-02018-6 - PMC - PubMed
-
- Hebert JS, Rehani M, Stiegelmar R. Osseointegration for Lower-Limb amputation: a systematic review of clinical outcomes. JBJS Rev. Oct. 2017;5(10):e10–10. 10.2106/JBJS.RVW.17.00037. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

