Restoring carboxypeptidase E rescues BDNF maturation and neurogenesis in aged brains
- PMID: 39872114
- PMCID: PMC11749474
- DOI: 10.1093/lifemedi/lnad015
Restoring carboxypeptidase E rescues BDNF maturation and neurogenesis in aged brains
Abstract
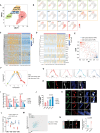
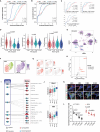
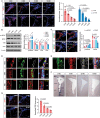
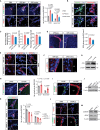
Adult neurogenesis declines with age due to the less functional neural stem cells (NSCs) and niches, but the underlying molecular bases for this impaired condition remain unclear. Here we analyzed >55,000 single-cell transcriptomes from two discrete neurogenic niches across the mouse lifespan, and identified new features and populations in NSCs, new markers, and neurogenic regional-specific alternations during aging. Intercellular communication analysis revealed defects in brain-derived neurotrophic factor (BDNF)-TrkB signaling cascade in old NSCs. Carboxypeptidase E (CPE) was found to be highly enriched in NSCs, and played a crucial role in mature/proBDNF balance and adult neurogenesis. Diminishment of CPE with aging resulted in impaired generation of BDNF, thus limiting the neurogenesis in old neurogenic niches. Restoring CPE expression markedly rescued the adult neurogenesis by increasing the production of mature BDNF, offering an attractive therapeutic strategy for the treatment of certain disorders in regions associated with constitutive neurogenesis.
Keywords: BDNF; adult neurogenesis; aging; carboxypeptidase E.
© The Author(s) 2023. Published by Oxford University Press on behalf of Higher Education Press.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Agomirs upregulating carboxypeptidase E expression rescue hippocampal neurogenesis and memory deficits in Alzheimer's disease.Transl Neurodegener. 2024 Apr 26;13(1):24. doi: 10.1186/s40035-024-00414-z. Transl Neurodegener. 2024. PMID: 38671492 Free PMC article.
-
Absence of Carboxypeptidase E/Neurotrophic Factor-Α1 in Knock-Out Mice Leads to Dysfunction of BDNF-TRKB Signaling in Hippocampus.J Mol Neurosci. 2017 May;62(1):79-87. doi: 10.1007/s12031-017-0914-0. Epub 2017 Apr 6. J Mol Neurosci. 2017. PMID: 28386642
-
Chromatin accessibility dynamics of neurogenic niche cells reveal defects in neural stem cell adhesion and migration during aging.Nat Aging. 2023 Jul;3(7):866-893. doi: 10.1038/s43587-023-00449-3. Epub 2023 Jul 13. Nat Aging. 2023. PMID: 37443352 Free PMC article.
-
Adult neural stem cells, neurogenic niches, and cellular therapy.Stem Cell Rev. 2006;2(3):213-9. doi: 10.1007/s12015-006-0049-0. Stem Cell Rev. 2006. PMID: 17625257 Review.
-
Role of Astrocytes in the Neurogenic Niches.Methods Mol Biol. 2019;1938:19-33. doi: 10.1007/978-1-4939-9068-9_2. Methods Mol Biol. 2019. PMID: 30617970 Review.
Cited by
-
Neurotrophic factor-α1/carboxypeptidase E controls progression and reversal of Alzheimer's disease pathogenesis in mice.Theranostics. 2025 Jan 13;15(6):2279-2292. doi: 10.7150/thno.99908. eCollection 2025. Theranostics. 2025. PMID: 39990227 Free PMC article.
-
Gut Microbiota Modulation by Lysozyme as a Key Regulator of Vascular Inflammatory Aging.Research (Wash D C). 2025 May 23;8:0704. doi: 10.34133/research.0704. eCollection 2025. Research (Wash D C). 2025. PMID: 40416362 Free PMC article.
-
Connexin 43 is downregulated in advanced Parkinson's disease in multiple brain regions which correlates with symptoms.Sci Rep. 2025 Mar 25;15(1):10250. doi: 10.1038/s41598-025-94188-7. Sci Rep. 2025. PMID: 40133513 Free PMC article.
-
Neurotrophic factor alpha 1 gene therapy in Alzheimer's disease: scope and advancements.Front Mol Neurosci. 2025 Apr 1;18:1518868. doi: 10.3389/fnmol.2025.1518868. eCollection 2025. Front Mol Neurosci. 2025. PMID: 40235693 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
