Harvestable tumour spheroids initiated in a gelatin-carboxymethyl cellulose hydrogel for cancer targeting and imaging with fluorescent gold nanoclusters
- PMID: 39872615
- PMCID: PMC11756458
- DOI: 10.1007/s44164-022-00033-w
Harvestable tumour spheroids initiated in a gelatin-carboxymethyl cellulose hydrogel for cancer targeting and imaging with fluorescent gold nanoclusters
Abstract
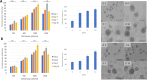
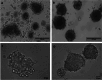
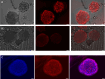
Cancer cell spheroids are the simplest 3D in vitro cancer models and have been extensively used for cancer research. More recently, models have been becoming complex, with the introduction of a matrix and non-cancer cell types to mimic specific tumour aspects. However, applying drugs or agents in matrix-embedded cancer spheroids can be problematic. Most matrices can impede and also bind drugs or visualizing agents non-specifically, in the vicinity of the embedded spheroids. This may interfere with imaging or further analysis without breaking apart the 3D model into its constituents. Here, we developed a combined gelatin-carboxymethyl cellulose (G-CMC) hydrogel for initiating cancer spheroids that enabled intact harvesting pre/post treatment for further investigation, such as targeting and imaging. We combined CMC (1.25%) and gelatin (2.5%) at 25 °C and initiated polymerisation after autoclaving (121 °C) to obtain a mechanical strength (sheer stress) of 38 Pas versus 1.28 Pas for CMC alone. These matrix conditions facilitated separation of the spheroids from the G-CMC, using low centrifugation (100 g). We described growth of colorectal and breast cancer spheroids within the G-CMC matrix (with average diameters of 220 mm and 180 μm for representative cell lines HT29 and MCF7 at 10 days, respectively). As the cancer cells express the surface biomarker calreticulin (CRT), we manufactured anti-calreticulin IgG (anti-CRT) conjugated to fluorescent gold nanoclusters (anti-CRT-AuNC) as a probe. We harvested cancer spheroids and incubated live with the nanoclusters. Imaging demonstrated strong binding of CRT-targeted AuNCs compared to control AuNCs. This novel model preserves cancer spheroid integrity upon isolation and is well suited for targeted imaging and drug delivery of cancer in 3D.
Keywords: 3D in vitro cancer model; Breast cancer spheroids; Calreticulin; Cancer targeting; Carboxymethyl cellulose; Colorectal cancer spheroids; Gelatin; Gold nanoclusters.
© The Author(s) 2022.
Conflict of interest statement
Conflict of interestThe authors declare no competing interests.
Figures



References
-
- Santini MT, Rainaldi G, Romano R, Ferrante A, Clemente S, Motta A, Indovina PL. MG-63 human osteosarcoma cells grown in monolayer and as three-dimensional tumor spheroids present a different metabolic profile: a (1)H NMR study. FEBS Lett. 2004;557:148–54. 10.1016/S0014-5793(03)01466-2. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
