The complexities of elexacaftor/tezacaftor/ivacaftor therapeutic drug monitoring in a person with cystic fibrosis and Mycobacterium abscessus pulmonary disease
- PMID: 39872799
- PMCID: PMC11770854
- DOI: 10.1080/20018525.2025.2458341
The complexities of elexacaftor/tezacaftor/ivacaftor therapeutic drug monitoring in a person with cystic fibrosis and Mycobacterium abscessus pulmonary disease
Abstract
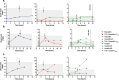
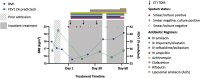
Therapeutic drug monitoring (TDM) of elexacaftor/tezacaftor/ivacaftor (ETI) remains challenging due to a lack of clarity around the parameters that govern ETI plasma concentrations, whilst the use of concomitant CYP3A inducers rifabutin and rifampicin is not recommended. We present the complexities of TDM for ETI performed in a person with cystic fibrosis and refractory Mycobacterium abscessus pulmonary disease. Utilising National Association of Testing Authorities (NATA) accredited assays and target considerations published by the Therapeutic Goods Administration (TGA), Australia, ETI plasma concentration variability was monitored over the course of an acute admission with added complexity from an antibiotic regimen including rifabutin, a moderate cytochrome P450 3A (CYP3A) inducer, and clofazimine, a mild CYP3A inhibitor. This case highlights the challenges surrounding ETI TDM in the context of acute severe illness, malnutrition, chronic infection, and drug-to-drug interactions. The marked clinical improvement seen, alongside sustained ETI plasma concentrations and suppressed sweat chloride levels on serial testing, provided reassurance of the use of ETI and rifabutin concomitantly in this case, and highlights the potential utility of TDM in helping guide clinical practice. Though a current barrier to the application of TDM includes ETI only being available as a fixed dose combination.
Keywords: Cystic Fibrosis; Kaftrio; Therapeutic drug monitoring; Trikafta; elexacaftor/tezacaftor/ivacaftor; non-tuberculous mycobacteria.
© 2025 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
-
- Hong E, Almond LM, Chung PS, et al. Physiologically based pharmacokinetic modeling to guide management of drug interactions between elexacaftor-tezacaftor-ivacaftor and antibiotics for the treatment of nontuberculous mycobacteria. Antimicrob Agents Chemother. 2022;66(11):e01104–5. doi: 10.1128/aac.01104-22 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
