BCG-Induced DNA Methylation Changes Improve Coronavirus Disease 2019 Vaccine Immunity Without Decreasing the Risk for Severe Acute Respiratory Syndrome Coronavirus 2 Infection
- PMID: 39872813
- PMCID: PMC11770274
- DOI: 10.1093/ofid/ofaf007
BCG-Induced DNA Methylation Changes Improve Coronavirus Disease 2019 Vaccine Immunity Without Decreasing the Risk for Severe Acute Respiratory Syndrome Coronavirus 2 Infection
Abstract
Background: The BCG vaccine induces trained immunity, an epigenetic-mediated increase in innate immune responsiveness. Therefore, this clinical trial evaluated if BCG-induced trained immunity could decrease coronavirus disease 2019 (COVID-19)-related frequency or severity.
Methods: A double-blind, placebo-controlled clinical trial of healthcare workers randomized participants to vaccination with BCG TICE or placebo (saline). Enrollment included 529 healthcare workers randomized to receive BCG or placebo. Primary analysis evaluated COVID-19 disease frequency, while secondary analysis evaluated coronavirus immunity in a subset of participants. Study enrollment ceased early in December 2020 following introduction of COVID-19-specific vaccines.
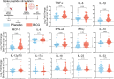
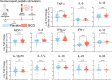
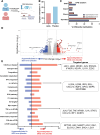
Results: Study enrollment was halted early, prior to reaching the targeted recruitment, and was not powered to detect a decrease in COVID-19 frequency. Symptomatic COVID-19 occurred in 21 of 263 and 10 of 266 participants in the BCG and placebo arms, respectively (P = .50, Fisher exact test). Participants vaccinated with BCG, but uninfected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), demonstrated increased coronavirus vaccine immunity (increase spike-inducible levels of tumor necrosis factor, interleukin 6, and interleukin 1β) 12 months after BCG vaccination compared to participants receiving placebo. Immune responsiveness to SARS-CoV-2 antigens correlated with BCG-induced DNA methylation changes.
Conclusions: Due to early study closure, the study was not powered to evaluate COVID-19 frequency. Secondary analysis demonstrated that 12 months following vaccination, BCG increased coronavirus vaccine immunity compared to those who did not receive BCG. This increase in COVID-19 vaccine immunity correlated with BCG-induced DNA methylation changes.
Keywords: BCG vaccine; COVID-19; DNA methylation; epigenetics; innate training.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. M. G. N. has a grant from the European Research Council Advanced Grant and the Netherlands Organization for Scientific Research (Spinoza Grant); serves as a consultant on the scientific advisory board of TTxD; holds 2 licensed patents related to nanobiological compositions for promoting trained immunity (US2020/0253884253884A1); and owns stock in TTxD, Lemba, and Biotrip. G. N. has Chancellor's funding as a Clinical Research Coordinator, with a 10% full-time equivalent as co-investigator. He also has 2 grants: one from CPRIT for the Gene-Environment-Lifestyle Interaction in Cancer Prevention, in which he is a co-investigator; and another from the US Department of Health and Human Services for Primary Care Training and Enhancement–Community Prevention and Maternal Health, for which he also serves as a co-investigator. All other authors report no potential conflicts of interest.
Figures

References
-
- Mackaness GB. Cellular resistance to infection. J Exp Med 1962; 116:381–406. - PubMed
-
- Arts RJW, Moorlag S, Novakovic B, et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe 2018; 23:89–100.e5. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

