Low-dose intranasal deferoxamine modulates memory, neuroinflammation, and the neuronal transcriptome in the streptozotocin rodent model of Alzheimer's disease
- PMID: 39872995
- PMCID: PMC11770042
- DOI: 10.3389/fnins.2024.1528374
Low-dose intranasal deferoxamine modulates memory, neuroinflammation, and the neuronal transcriptome in the streptozotocin rodent model of Alzheimer's disease
Abstract
Introduction: Intranasal (IN) deferoxamine (DFO) has emerged over the past decade as a promising therapeutic in preclinical experiments across neurodegenerative and neurovascular diseases. As an antioxidant iron chelator, its mechanisms are multimodal, involving the binding of brain iron and the consequent engagement of several pathways to counter pathogenesis across multiple diseases. We and other research groups have shown that IN DFO rescues cognitive impairment in several rodent models of Alzheimer Disease (AD).
Methods: This study was designed to probe dosing regimens to inform future clinical trials, while exploring mechanisms within the intracerebroventricular (ICV) streptozotocin (STZ) model.
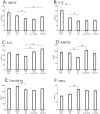
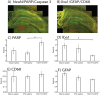
Results: Five weeks of daily IN dosing of Long Evans rats with 15 μL of a 1% (0.3 mg), but not 0.1% (0.03 mg), solution of DFO rescued cognitive impairment caused by ICV STZ administration as assessed with the Morris Water Maze (MWM) test of spatial memory and learning. Furthermore, IN DFO modulated several aspects of the neuroinflammatory milieu of the ICV STZ model, which was assessed through a novel panel of brain cytokines and immunohistochemistry. Using RNA-sequencing and pathway analysis, STZ was shown to induce several pathways of cell death and neuroinflammation, and IN DFO engaged multiple transcriptomic pathways involved in hippocampal neuronal survival.
Discussion: To our knowledge this study is the first to assess the transcriptomic pathways and mechanisms associated with either the ICV STZ model or DFO treatment, and the first to demonstrate efficacy at this low dose.
Keywords: Alzheimer’s disease; deferoxamine; intranasal; neuroinflammation; streptozotocin; transcriptome.
Copyright © 2025 Fine, Kosyakovsky, Bowe, Faltesek, Stroebel, Abrahante, Kelly, Thompson, Westby, Robertson, Frey and Hanson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

