TP53 Mutations in Myeloproliferative Neoplasms: Context-Dependent Evaluation of Prognostic Relevance
- PMID: 39873146
- PMCID: PMC11886478
- DOI: 10.1002/ajh.27609
TP53 Mutations in Myeloproliferative Neoplasms: Context-Dependent Evaluation of Prognostic Relevance
Abstract
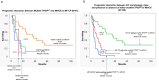
The clinical relevance of TP53 mutations (TP53 MUT ) in myeloproliferative neoplasms (MPN) and their prognostic interaction with MPN subtype designation has not been systematically studied. In the current study, 114 patients with MPN harboring TP53 MUT (VAF ≥ 2%) were evaluated for overall survival (OS), calculated from the time of TP53 MUT detection: chronic phase myelofibrosis (MF-CP; N = 61); blast-phase (MPN-BP; N = 31) or accelerated-phase (MPN-AP; N = 16) MPN, and polycythemia vera/essential thrombocythemia (PV/ET; N = 6). Sixty-five (57%) patients harbored International Consensus Classification (ICC)-defined multihit TP53 MUT and 56 (49%) monosomal/complex karyotype (MK/CK). Majority of MPN-BP (90%) and MPN-AP (81%) while 39% of MF-CP and none of PV/ET patients harbored multihit TP53 MUT . OS in MPN-BP and MPN-AP was equally dismal (median 6 vs. 4.5 months, respectively; p = 1.0), regardless of multihit configuration (p = 0.44), while OS in TP53 MUT MPN-BP/AP (N = 47; median 4 months) was inferior to that of a separate cohort (N = 49) with TP53 wild-type MPN-BP/AP (median 11 months; p < 0.01). OS in MF-CP was significantly shorter with multihit versus non-multihit TP53 MUT (median 10 vs. 35 months; HR 2.9; p < 0.01), independent of other MF-relevant genetic risk factors, including ASXL1/SRSF2/U2AF1 mutations. Multihit TP53 MUT was also associated with inferior survival following allogeneic stem cell transplant (ASCT): median 9 months versus "not reached" in patients with (N = 9) versus without (N = 8) multihit TP53 MUT (p < 0.01). The presence of multihit or non-multihit TP53 MUT in MPN-BP/AP or multihit TP53 MUT in MF-CP is associated with exceptionally poor prognosis and justifies inclusion into the ICC category of "myeloid neoplasms with mutated TP53." By contrast, detection of non-multihit TP53 MUT, by itself, might not endanger short-term survival in MF-CP, PV, or ET.
Keywords: ICC; WHO; karyotype; prognosis; survival.
© 2025 The Author(s). American Journal of Hematology published by Wiley Periodicals LLC.
Conflict of interest statement
A.T. nothing to disclose; M.A. nothing to disclose; G.G.L. nothing to disclose; S.F. nothing to disclose; K.H.B. nothing to disclose; A.A.K. research support to institution: Novartis, Onconova, ALX Oncology, BMS, Celgene, H3Biomedicine/Hemavant, Aprea, Astex, MedImmune. Consultancy: Novartis; J.F. nothing to disclose; J.P. nothing to disclose; T.B. advisory board for Takeda, Pfizer, Morphosys, and Syndex; M.M.P. received research funding from Kura Oncology, Stemline therapeutics, Epigenetix, Solutherapeutics, Polaris and has served on the advisory board for AstraZeneca and SOBI pharmaceuticals; K.K.R. nothing to disclose; R.H. nothing to disclose; C.J.Z.M. nothing to disclose; M.S.: research funding to the institution from Astellas, AbbVie, Celgene, KURA Oncology, and Marker Therapeutics; A.O. nothing to disclose; D.A.A. nothing to disclose; A.P. nothing to disclose; A.M.V. advisory Board of Novartis, Incyte, BMS, Italfarmaco and received fees for lectures from BMS, Novartis, Blueprint, Abbvie, Italfarmaco; D.H. member of the board of directors or advisory committees of AbbVie and Novartis; N.G. advisory board to DISC Medicine and Agios; P.G. advisory Board of Novartis, Incyte, GSK and received fees for lectures from GSK, Novartis and abbvie.
Figures



References
-
- Rucker F. G., Schlenk R. F., Bullinger L., et al., “TP53 Alterations in Acute Myeloid Leukemia With Complex Karyotype Correlate With Specific Copy Number Alterations, Monosomal Karyotype, and Dismal Outcome,” Blood 119 (2012): 2114–2121. - PubMed
-
- Weinberg O. K., Porwit A., Orazi A., et al., “The International Consensus Classification of Acute Myeloid Leukemia,” Virchows Archiv 482 (2023): 27–37. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

