Gestational trophoblastic disease: understanding the molecular mechanisms of placental tumours
- PMID: 39873178
- PMCID: PMC11810044
- DOI: 10.1242/dmm.052010
Gestational trophoblastic disease: understanding the molecular mechanisms of placental tumours
Abstract
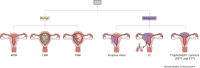
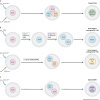
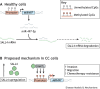
Gestational trophoblastic disease (GTD) describes a group of rare benign and cancerous lesions originating from the trophoblast cells of the placenta. These neoplasms are unconventional entities, being one of the few instances in which cancer develops from the cells of another organism, the foetus. Although this condition was first described over 100 years ago, the specific genetic and non-genetic drivers of this disease remain unknown to this day. However, recent findings have provided valuable insights into the potential mechanisms underlying this rare condition. Unlike previous reviews focused primarily on the clinical and diagnostic aspects of disease development, this Review consolidates the latest research concerning the role of genetics, epigenetics and microRNAs in the initiation and progression of GTD. By examining GTD from a molecular perspective, this Review provides a unique framework for understanding the pathogenesis and progression of this rare disease.
Keywords: Choriocarcinoma; Gestational trophoblastic disease; Gestational trophoblastic neoplasia; Molar pregnancy; Placental tumour.
© 2025. Published by The Company of Biologists.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures
Similar articles
-
Pathology of Gestational Trophoblastic Disease (GTD).Hematol Oncol Clin North Am. 2024 Dec;38(6):1191-1217. doi: 10.1016/j.hoc.2024.08.017. Epub 2024 Sep 24. Hematol Oncol Clin North Am. 2024. PMID: 39322461 Review.
-
Pathology of gestational trophoblastic disease (GTD).Best Pract Res Clin Obstet Gynaecol. 2021 Jul;74:3-28. doi: 10.1016/j.bpobgyn.2021.02.005. Epub 2021 Mar 31. Best Pract Res Clin Obstet Gynaecol. 2021. PMID: 34219021 Review.
-
Advances in diagnostics and management of gestational trophoblastic disease.Radiol Oncol. 2022 Oct 27;56(4):430-439. doi: 10.2478/raon-2022-0038. eCollection 2022 Dec 1. Radiol Oncol. 2022. PMID: 36286620 Free PMC article. Review.
-
Gestational trophoblastic disease- rare, sometimes dramatic, and what we know so far.Semin Diagn Pathol. 2022 May;39(3):228-237. doi: 10.1053/j.semdp.2022.03.002. Epub 2022 Mar 22. Semin Diagn Pathol. 2022. PMID: 35400536 Review.
-
Gestational Trophoblastic Tumors: A Timely Review of Diagnostic Pathology.Arch Pathol Lab Med. 2019 Jan;143(1):65-74. doi: 10.5858/arpa.2018-0234-RA. Epub 2018 Nov 8. Arch Pathol Lab Med. 2019. PMID: 30407075 Review.
Cited by
-
Hyperthyroidism Associated with Gestational Trophoblastic Neoplasia: Systematic Literature Review and Pathways Analysis.Cancers (Basel). 2025 Apr 22;17(9):1398. doi: 10.3390/cancers17091398. Cancers (Basel). 2025. PMID: 40361325 Free PMC article. Review.
References
-
- Ahmed, M. N., Kim, K., Haddad, B., Berchuck, A. and Qumsiyeh, M. B. (2000). Comparative genomic hybridization studies in hydatidiform moles and choriocarcinoma: amplification of 7q21-q31 and loss of 8p12-p21 in choriocarcinoma. Cancer Genet. Cytogenet. 116, 10-15. 10.1016/s0165-4608(99)00103-x - DOI - PubMed
-
- Allias, F., Mechtouf, N., Gaillot-Durand, L., Hoffner, L., Hajri, T., Devouassoux-Shisheboran, M., Massardier, J., Golfier, F., Bolze, P.-A., Surti, U.et al. (2020). A novel NLRP7 protein-truncating mutation associated with discordant and divergent p57 immunostaining in diploid biparental and triploid digynic moles. Virchows Arch. 477, 309-315. 10.1007/s00428-020-02769-w - DOI - PubMed
-
- Boyero, L., Noguera-Uclés, J. F., Castillo-Peña, A., Salinas, A., Sánchez-Gastaldo, A., Alonso, M., Benedetti, J. C., Bernabé-Caro, R., Paz-Ares, L. and Molina-Pinelo, S. (2023). Aberrant methylation of the imprinted C19MC and MIR371-3 clusters in patients with non-small cell lung cancer. Cancers 15, 1466. 10.3390/cancers15051466 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

