Investigation into the effect of phenylalanine gating on anaerobic haem breakdown using the energy landscape approach
- PMID: 39873208
- PMCID: PMC11773379
- DOI: 10.1002/pro.5243
Investigation into the effect of phenylalanine gating on anaerobic haem breakdown using the energy landscape approach
Abstract
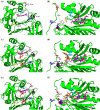
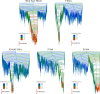
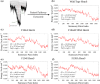
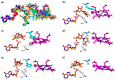
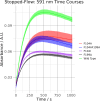
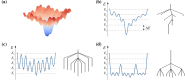
We have recently demonstrated a novel anaerobic NADH-dependent haem breakdown reaction, which is carried out by a range of haemoproteins. The Yersinia enterocolitica protein, HemS, is the focus of further research presented in the current paper. Using conventional experimental methods, bioinformatics, and energy landscape theory (ELT), we provide new insight into the mechanism of the novel breakdown process. Of particular interest is the behavior of a double phenylalanine gate, which opens and closes according to the relative situations of haem and NADH within the protein pocket. This behavior suggests that the double phe-gate fulfills a regulatory role within the pocket, controlling the access of NADH to haem. Additionally, stopped-flow spectroscopy results provide kinetic comparisons between the wild-type and the selected mutants. We also present a fully resolved crystal structure for the F104AF199A HemS monomer, including its extensive loop, the first such structure to be completely resolved for HemS or any of its close homologues. The energy landscapes approach provided key information regarding the gating strategy employed by HemS, compensating for current limitations with conventional biophysical and molecular dynamics approaches. We propose that ELT become more widely used in the field, particularly in the investigation of the dynamics and interactions of weak-binding ligands, and for gating features, within protein cavities.
Keywords: HemS; Yersinia enterocolitica; biophysics; energy landscapes; heme; nicotinamide adenine dinucleotide (NADH); protein dynamics; protein gates; residue‐mediated regulation.
© 2025 The Author(s). Protein Science published by Wiley Periodicals LLC on behalf of The Protein Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Crystallization and preliminary X-ray diffraction analysis of the haem-binding protein HemS from Yersinia enterocolitica.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2005 Aug 1;61(Pt 8):802-5. doi: 10.1107/S1744309105023523. Epub 2005 Jul 30. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2005. PMID: 16511163 Free PMC article.
-
Haem, flavin and oxygen interactions in Hmp, a flavohaemoglobin from Escherichia coli.Biochem Soc Trans. 1994 Aug;22(3):709-13. doi: 10.1042/bst0220709. Biochem Soc Trans. 1994. PMID: 7821669 No abstract available.
-
Combining experiment and energy landscapes to explore anaerobic heme breakdown in multifunctional hemoproteins.Phys Chem Chem Phys. 2024 Jan 3;26(2):695-712. doi: 10.1039/d3cp03897a. Phys Chem Chem Phys. 2024. PMID: 38053511 Review.
-
Insights into heme-based O2 sensing from structure-function relationships in the FixL proteins.J Inorg Biochem. 2005 Apr;99(4):963-77. doi: 10.1016/j.jinorgbio.2005.02.016. J Inorg Biochem. 2005. PMID: 15811514
-
Bacterial heme oxygenases.Antioxid Redox Signal. 2004 Oct;6(5):825-34. doi: 10.1089/ars.2004.6.825. Antioxid Redox Signal. 2004. PMID: 15345142 Review.
References
-
- Allen RJ, Valeriani C, Rein Ten Wolde P. Forward flux sampling for rare event simulations. J Phys Cond Matt. 2009;152:060901. - PubMed
-
- André AC, Debande L, Marteyn BS. The selective advantage of facultative anaerobes relies on their unique ability to cope with changing oxygen levels during infection. Cell Microbiol. 2021;23:1–8. - PubMed
-
- Arroyo‐Mañez P, Bikiel DE, Boechi L, Capece L, Di Lella S, Estrin DA, et al. Protein dynamics and ligand migration interplay as studied by computer simulation. Biochim Biophys Acta Proteins Proteom. 2011;1814:1054–1064. - PubMed
-
- Becker OM, Karplus M. The topology of multidimensional potential energy surfaces: theory and application to peptide structure and kinetics. J Chem Phys. 1997;106:1495–1517.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

