Population Kinetics and Protein Profiles of Co-Cultured Adult and Fetus Rabbit Bladder Smooth Muscle Cells
- PMID: 39873448
- PMCID: PMC11883674
- DOI: 10.5152/tud.2025.24120
Population Kinetics and Protein Profiles of Co-Cultured Adult and Fetus Rabbit Bladder Smooth Muscle Cells
Abstract
Objective: Bladder tissue models have been developed using smooth muscle cells (SMCs) on various scaffolds to mimic bladder morphology and physiology. This study investigates the effects of co-culturing fetal and adult SMCs on growth properties and protein profiles to understand cellular interactions and population kinetics.
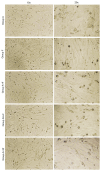
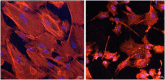
Methods: Bladder tissue samples from 10 adult and 10 fetal New Zealand rabbits were divided into 5 groups: adult SMCs (A), fetal SMCs (F), 50%A+50%F (A+F), 75%A+25%F (3A+F), and 25%A+75%F (A+3F). Population doubling time (PDT) of 3 × 106 cells from each group was measured after 48 and 72 hours. Protein concentrations were estimated by spectrophotometric analysis and analyzed via SDS-PAGE gel electrophoresis. Cells exhibited typical SMC morphology, confirmed by positive staining for α-SMA and MYH11.
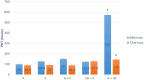
Results: Median cell counts of single cultures were significantly higher than co-cultures (P < .05), but cell viability was comparable (P > .05). Population doubling time at 72 hours for A, F, A+F, 3A+F, and A+3F were 89.4, 92.0, 89.4, 127.9, and 145.0 hours, respectively. Protein concentrations were similar between fetal and adult co-cultures (P > .05). Electrophoresis at 48 hours revealed a unique 80kDa band in adult cells and a 32kDa band in co-cultured cells.
Conclusion: Co-culturing resulted in increased PDT, altered protein concentrations, and changes in protein profiles, while each cell population maintained its phenotype. Fetal bladder SMCs maintained their morphology and viability when co-cultured with adult SMCs, resulting in a significant limitation in the cumulative proliferation rate. This may be dependent on alterations of protein profiles of adult and fetal SMCs promoted by rearrangements in co-cultures.
Keywords: Bladder smooth muscle cells; bladder tissue model; population kinetics; protein profiles.
Conflict of interest statement
Figures

References
LinkOut - more resources
Full Text Sources
