Aspirin Plus Rivaroxaban Versus Rivaroxaban Alone for the Prevention of Venous Stent Thrombosis Among Patients With Post-Thrombotic Syndrome: The Multicenter, Multinational, Randomized, Open-Label ARIVA Trial
- PMID: 39874026
- PMCID: PMC11932446
- DOI: 10.1161/CIRCULATIONAHA.124.073050
Aspirin Plus Rivaroxaban Versus Rivaroxaban Alone for the Prevention of Venous Stent Thrombosis Among Patients With Post-Thrombotic Syndrome: The Multicenter, Multinational, Randomized, Open-Label ARIVA Trial
Abstract
Background: In patients with post-thrombotic syndrome, stent recanalization of iliofemoral veins or the inferior vena cava can restore venous patency and improve functional outcomes. The risk of stent thrombosis is particularly increased during the first 6 months after intervention. The ARIVA trial (Aspirin Plus Rivaroxaban Versus Rivaroxaban Alone for the Prevention of Venous Stent Thrombosis in Patients With PTS) tested whether 100 mg of daily aspirin plus 20 mg of rivaroxaban is superior to 20 mg of rivaroxaban alone to prevent stent thrombosis within 6 months after stent placement for post-thrombotic syndrome.
Methods: In this multinational, academic, open-label, independently adjudicated trial, patients with a Villalta score >4 points and a stenosis or occlusion of the inferior vena cava, iliac veins, or common femoral vein successfully treated with venous stent placement were randomized in a 1:1 fashion to the study groups. Key exclusion criteria included <18 or >75 years of age, contraindications to anticoagulant use, or acute venous thrombosis <3 months. The primary efficacy outcome was the composite of no occlusion in the treated segment assessed at serial duplex ultrasound examinations or no reintervention needed to maintain patency within 6 months. Secondary outcomes, including Villalta score, quality of life, and safety outcomes, were also assessed. The study was registered at ClinicalTrials.gov (NCT04128956).
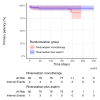
Results: From 2020 through 2022, 172 patients were screened, 169 were randomized, and 162 were included in the full analysis set, receiving either aspirin plus rivaroxaban (n=80) or rivaroxaban alone (n=82) for 6 months. Mean±SD age was 42.8±14.7 years; 103 patients (60.9%) were women; 154 patients (97.5%) were White; and leg ulcers were present in 7% of patients. The primary patency rate at 6 months was 94.8% versus 92.4% (absolute risk difference, 2.4% [95% CI, -13.6 to 18.0]), respectively. The mean±SD decrease in the Villalta score for the affected leg (without ulcer) from baseline to 6 months was -6.7±4.4 and -7.0±5.2 points (P=0.36), respectively. There were no differences in other outcomes or quality of life at 6 months. No major bleeding occurred.
Conclusions: The overall primary patency rate during the first 6 months after endovascular intervention for post-thrombotic syndrome was higher than expected and comparable between patients receiving aspirin combined with rivaroxaban and those receiving rivaroxaban alone.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT04128956.
Keywords: aspirin; endovascular procedures; post-thrombotic syndrome; rivaroxaban; thrombosis.
Conflict of interest statement
Dr Barco received grant support from Concept Medical, Bayer, Boston Scientific, Sanofi, Novartis, and Daiichi Sankyo. He also received consultancy or lecturer fees from Boston Scientific, Concept Medical, Daiichi Sankyo, Viatris, and Sanofi. Dr Jalaie received consultancy or lecturer fees from Medtronic, Betcon Dickenson, Philips, Bentley, Optimed, and Abbott. He also received grant support from Medtronic, Betcon Dickenson, Cook, Bentely, Optimed, Boston Scientific, and Abbott. Dr Sebastian, S. Wolf, Dr Fumagalli, and Dr Lichtenberg report no conflicts. Dr Erbel received consultancy or lecturer fees from Boston Scientific, Philips, and Bristol Myers Squibb. Dr Schlager received consultancy or lecturer fees from Becton Dickenson, Biotronik, Philips, and Optimed. Dr Kucher received grant support from Concept Medical, Bayer, Becton Dickenson, Boston Scientific, and Medtronic. He also received consultancy or lecturer fees from Boston Scientific and Becton Dickenson. Dr Zeller received honoraria from Biotronik, Boston Scientific, Cook Medical, Cordis, Gore, Medtronic, Shockwave, Veryan, BD Bard, Reflow Medical, and Acotec.
Figures
References
-
- Wolf S, Barco S, Di Nisio M, Mahan CE, Christodoulou KC, Ter Haar S, Konstantinides S, Kucher N, Klok FA, Cannegieter SC, et al. . Epidemiology of deep vein thrombosis. Vasa. 2024;53:298–307. doi: 10.1024/0301-1526/a001145 - PubMed
-
- Brown C, Tokessy L, Delluc A, Carrier M. Risk of developing post thrombotic syndrome after deep vein thrombosis with different anticoagulant regimens: a systematic review and pooled analysis. Thromb Res. 2024;240:109057. doi: 10.1016/j.thromres.2024.109057 - PubMed
-
- Khider L, Planquette B, Smadja DM, Sanchez O, Rial C, Goudot G, Messas E, Mirault T, Gendron N. Acute phase determinant of post-thrombotic syndrome: a review of the literature. Thromb Res. 2024;238:11–18. doi: 10.1016/j.thromres.2024.04.004 - PubMed
-
- Silva JC, Constancio V, Lima P, Nunes C, Silva E, Anacleto G, Fonseca M. Determinants of quality of life in patients with post-thrombotic syndrome. Ann Vasc Surg. 2022;85:253–261. doi: 10.1016/j.avsg.2022.03.002 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

