Net Benefit of Early Anticoagulation for Stroke With Atrial Fibrillation: Post Hoc Analysis of the ELAN Randomized Clinical Trial
- PMID: 39874037
- PMCID: PMC11775740
- DOI: 10.1001/jamanetworkopen.2024.56307
Net Benefit of Early Anticoagulation for Stroke With Atrial Fibrillation: Post Hoc Analysis of the ELAN Randomized Clinical Trial
Abstract
Importance: The net clinical effect of early vs later direct oral anticoagulant (DOAC) initiation after atrial fibrillation-associated ischemic stroke is unclear.
Objective: To investigate whether early DOAC treatment is associated with a net clinical benefit (NCB).
Design, setting, and participants: This was a post hoc analysis of the Early Versus Late Initiation of Direct Oral Anticoagulants in Post-Ischaemic Stroke Patients With Atrial Fibrillation (ELAN) open-label randomized clinical trial conducted across 103 sites in 15 countries in Europe, the Middle East, and Asia between November 6, 2017, and September 12, 2022, with a 90-day follow-up. Participants included patients with atrial fibrillation-associated acute ischemic stroke, excluding those with therapeutic anticoagulation at stroke onset or with severe hemorrhagic transformation of the ischemic infarct.
Intervention: Early DOAC initiation (<48 hours after minor and moderate stroke, 6-7 days after major stroke) vs later initiation (3-4 days after minor stroke, 6-7 days after moderate stroke, and 12-14 days after major stroke).
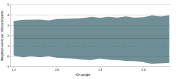
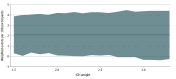
Main outcomes and measures: The main measure was the NCB of early treatment over later treatment, calculated by subtracting the weighted rate of excess bleeding events (major extracranial or intracranial hemorrhage) attributable to early treatment from the rate of excess ischemic events (recurrent stroke or systemic embolism) possibly prevented by early treatment within 30 days (main analysis) or 90 days (ancillary analysis). An established weighting scheme was used to account for the different clinical impact of bleeding relative to ischemic outcomes. Event rates were derived from adjusted logistic models. The analysis included all evaluable randomized ELAN participants.
Results: Of the original 2013 ELAN participants, 1966 were eligible for analysis (977 [49.7%] assigned to early DOAC initiation, 989 [50.3%] assigned to later DOAC initiation; median [IQR] age 77 [70-84] years; 1075 [54.7%] male). The 30-day NCB of early treatment over later treatment ranged from 1.73 (95% CI, 0.06-3.40) to 1.72 (95% CI, -0.63 to 3.98) weighted events possibly prevented per 100 participants for intracranial hemorrhage weights 1.5 to 3.3. The 90-day NCB ranged from 2.16 (95% CI, 0.30-3.87) to 2.14 (95% CI, -0.26 to 4.41) weighted events per 100 participants.
Conclusions and relevance: This post hoc analysis of a randomized clinical trial estimated a sizeable NCB of early anticoagulation for patients after atrial fibrillation-associated ischemic stroke. Although estimates cannot exclude the possibility of no benefit or small net harm, the findings suggest that early treatment may be more favorable.
Trial registration: ClinicalTrials.gov Identifier: NCT03148457.
Conflict of interest statement
Figures
References
-
- Oldgren J, Åsberg S, Hijazi Z, Wester P, Bertilsson M, Norrving B; National TIMING Collaborators . Early versus delayed non–vitamin k antagonist oral anticoagulant therapy after acute ischemic stroke in atrial fibrillation (TIMING): a registry-based randomized controlled noninferiority study. Circulation. 2022;146(14):1056-1066. doi: 10.1161/CIRCULATIONAHA.122.060666 - DOI - PMC - PubMed
-
- Werring DJ, Dehbi HM, Ahmed N, et al. ; OPTIMAS investigators . Optimal Timing of Anticoagulation After Acute Ischaemic Stroke with atrial fibrillation (OPTIMAS): a multicentre, blinded-endpoint, phase 4, randomised controlled trial. Lancet. 2024;404(10464):1731-1741. doi: 10.1016/S0140-6736(24)02197-4 - DOI - PubMed

