Fibroblast growth factor-inducible 14 regulates satellite cell self-renewal and expansion during skeletal muscle repair
- PMID: 39874107
- PMCID: PMC11949035
- DOI: 10.1172/jci.insight.187825
Fibroblast growth factor-inducible 14 regulates satellite cell self-renewal and expansion during skeletal muscle repair
Abstract
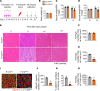
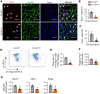
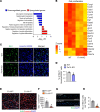
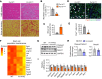
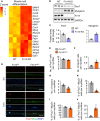
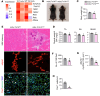
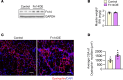
Skeletal muscle regeneration in adults is predominantly driven by satellite cells. Loss of satellite cell pool and function leads to skeletal muscle wasting in many conditions and disease states. Here, we demonstrate that the levels of fibroblast growth factor-inducible 14 (Fn14) were increased in satellite cells after muscle injury. Conditional ablation of Fn14 in Pax7-expressing satellite cells drastically reduced their expansion and skeletal muscle regeneration following injury. Fn14 was required for satellite cell self-renewal and proliferation as well as to prevent precocious differentiation. Targeted deletion of Fn14 inhibited Notch signaling but led to the spurious activation of STAT3 signaling in regenerating skeletal muscle and in cultured muscle progenitor cells. Silencing of STAT3 improved proliferation and inhibited premature differentiation of Fn14-deficient satellite cells. Furthermore, conditional ablation of Fn14 in satellite cells exacerbated myopathy in the mdx mouse model of Duchenne muscular dystrophy (DMD), whereas its overexpression improved the engraftment of exogenous muscle progenitor cells into the dystrophic muscle of mdx mice. Altogether, our study highlights the crucial role of Fn14 in the regulation of satellite cell fate and function and suggests that Fn14 can be a potential molecular target to improve muscle regeneration in muscular disorders.
Keywords: Cell biology; Muscle biology; Neuromuscular disease; Signal transduction; Skeletal muscle; Stem cells.
Conflict of interest statement
Figures


Update of
-
Fibroblast growth factor-inducible 14 regulates satellite cell self-renewal and expansion during skeletal muscle repair.bioRxiv [Preprint]. 2025 Jan 2:2024.10.06.616900. doi: 10.1101/2024.10.06.616900. bioRxiv. 2025. Update in: JCI Insight. 2025 Jan 28;10(5):e187825. doi: 10.1172/jci.insight.187825. PMID: 39803454 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

