Histological differences among thrombi in thrombotic diseases
- PMID: 39874150
- PMCID: PMC11957440
- DOI: 10.1097/MOH.0000000000000860
Histological differences among thrombi in thrombotic diseases
Abstract
Purpose of review: This review aims to summarize the histological differences among thrombi in acute myocardial infarction, ischemic stroke, venous thromboembolism, and amniotic fluid embolism, a newly identified thrombosis.
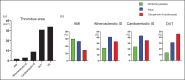
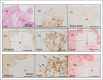
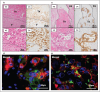
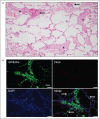
Recent findings: Acute coronary thrombi have a small size, are enriched in platelets and fibrin, and show the presence of fibrin and von Willebrand factor, but not collagen, at plaque rupture sites. Symptomatic deep vein thrombi are large and exhibit various phases of time-dependent histological changes. Cancer-associated venous thromboemboli contain invasive cancer cells that penetrate the vascular walls, and small cancer cell aggregates are observed within the thrombi. The thrombus composition in atherosclerotic and cardioembolic ischemic strokes varies from case to case, while the thrombi in cancer-associated ischemic stroke are rich in platelets and fibrin. A pathological study on amniotic fluid embolism identified uterine vein thrombi and massive platelet-rich microthrombi in the lungs.
Summary: Atherothrombus formation is induced by plaque disruption and may occlude a narrow lumen within a short time. Venous thrombi may grow to a large size in a multistage or chronic manner. Cancer cells can directly contribute to venous thrombus formation. The thrombus formation in amniotic fluid embolism may explain the occurrence of consumptive coagulopathy and cardiopulmonary collapse.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Figures



References
-
- Kelly RV, Cohen MG, Stouffer GA. Mechanical thrombectomy options in complex percutaneous coronary interventions. Catheter Cardiovasc Interv 2006; 68:917–928. - PubMed
-
- Yunoki K, Naruko T, Sugioka K, et al. . Erythrocyte-rich thrombus aspirated from patients with ST-elevation myocardial infarction: association with oxidative stress and its impact on myocardial reperfusion. Eur Heart J 2012; 33:1480–1490. - PubMed
-
- Riegger J, Byrne RA, Joner M, et al. . Histopathological evaluation of thrombus in patients presenting with stent thrombosis. A multicenter European study: a report of the prevention of late stent thrombosis by an interdisciplinary global European effort consortium. Eur Heart J 2016; 37:1538–1549. - PMC - PubMed
-
- Takahashi M, Yamashita A, Moriguchi-Goto S, et al. . Critical role of von Willebrand factor and platelet interaction in venous thromboembolism. Histol Histopathol 2009; 24:1391–1398. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

